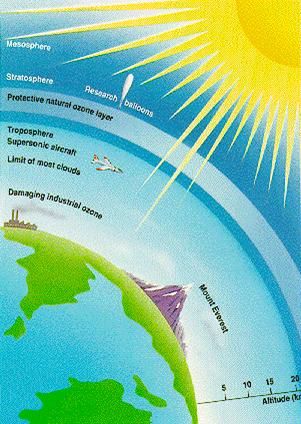
What is ozone?
The ozone layer is the part of the Earth’s atmosphere which contains relatively high concentrations of ozone gas, which is an inorganic molecule with the chemical formula O3.In the stratosphere,ozone blocks out the sun’s ultraviolet Ray’s and is a life saver.
The ozone layer in the stratosphere absorbs a portion of the radiation from the sun,preventing it from reaching the planet’s surface.Most importantly,it absorbs the portion of UV light called UVB.It has been linked to many harmful effects like skin cancers,cataracts,and harm to some crops and marine life.
Ozone as a natural sun block
The ozone layer is like the Earth’s sunscreen,as it absorbs some of the sun’s radiation hitting our place. Without the ozone, radiation from the sun would reach earth directly,damaging DNA of many plants,animals and even human beings.Skin cancerrates would be on the rise .
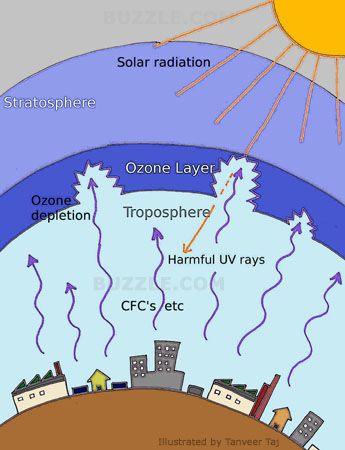
The electromagnetic radiation emitted from the sun includes ultraviolet radiation,which is potentially harmful to most living things since it can damage DNA.The ozone layer screens out the sun’s harmful ultraviolet radiation.Even 1 percent reduction in the amount of ozone in the upper stratosphere causes a measurable increase in the ultraviolet radiation thet reaches the earth surface.If there was no ozone at all,the amount of ultraviolet radiation reaching us would be very high.All living things would suffer radiation burns,unless they were underground or in the sea.
In the stratosphere,small amount of ozone are constantly being made by the action of sunlight on oxygen.At the same time,ozone is being broken down by natural processes.The total amount of ozone usually stays constant because it’s formation and destruction occur at about the same rate.But human activity has recently changed the natural balance.
When chlorine and bromine atoms come into contact with ozone in the stratosphere, they destroy ozone molecules. One chlorine atom can destroy over 100,000 ozone molecules before it is removed from the stratosphere. Ozone can be destroyed more quickly than it is naturally created.Some manufactured substances such as chlorofluorocarbons and hydrochlorofluorocarbons can destroy stratosphere ozone much faster than it is formed.
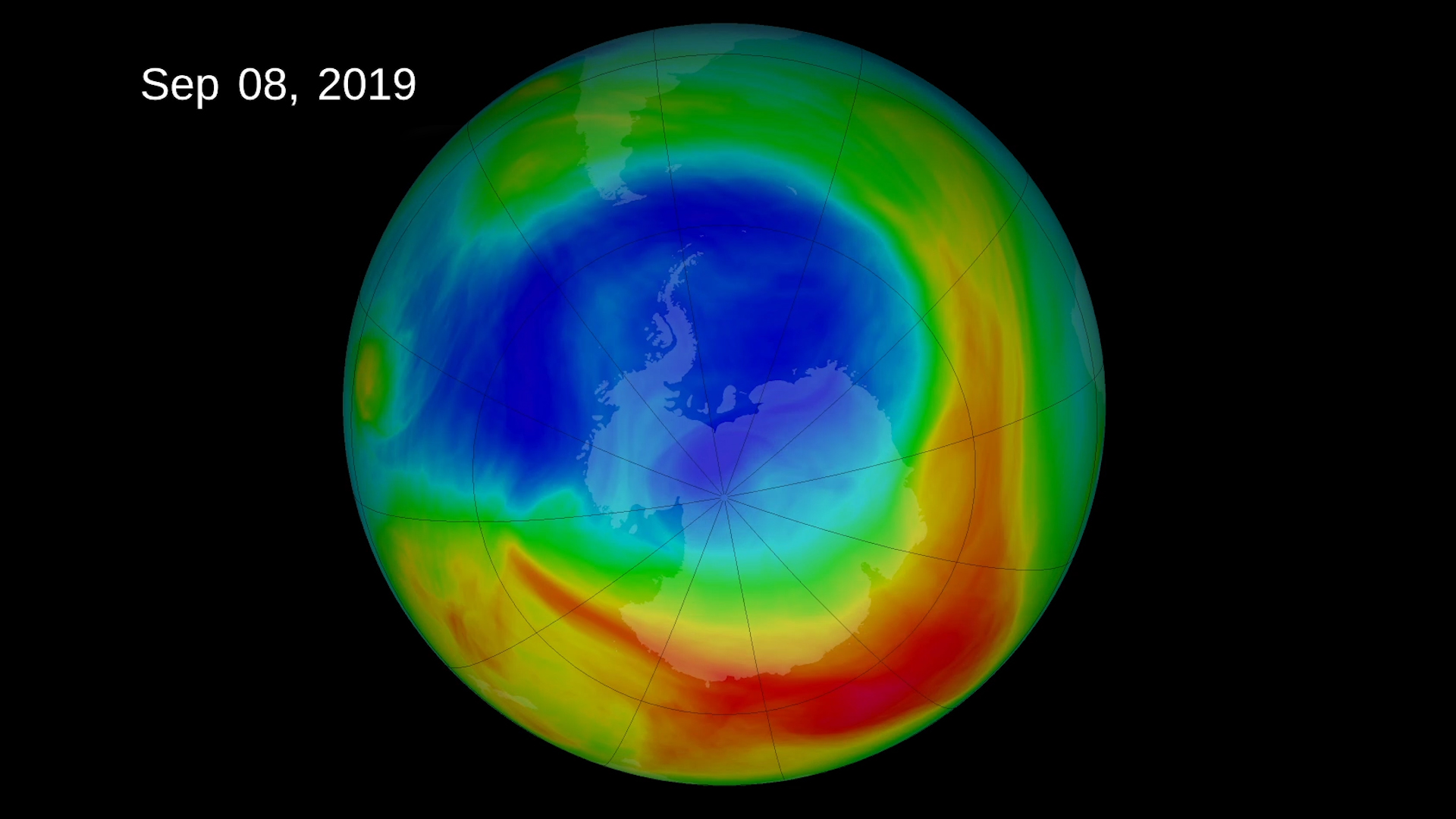
Ozone hole
Ozone layer depletion is the thinning of the ozone layer present in the upper atmosphere. The loss of ozone was first detected in the stratosphere over the Antarctic.Tye part of the atmosphere where ozone is most depleted is referred as ozone hole.But it is not a real hole just a vast region of the upper atmosphere where there is less ozone than elsewhere.
Ozone poor air can spread out from the polar regions and move above other areas.
Reasons for the ozone hole in Antarctica
The ozone hole formed over Antarctica is due to compounds of chlorine and bromine formed in the atmosphere.Most of the chlorine and half of the bromine in the stratosphere comes from human activities.The chlorofluorocarbons released due to human activities get transported up into the upper stratosphere.
The most common ozone depleting substances are chlorofluorocarbons or freon gases, certain bromine compounds, nitrogen oxides and methyl bromide.These are released from air conditioners,freezers,foam insulations,aerosol products, industrial solvents,fire extinguishers and pesticides.
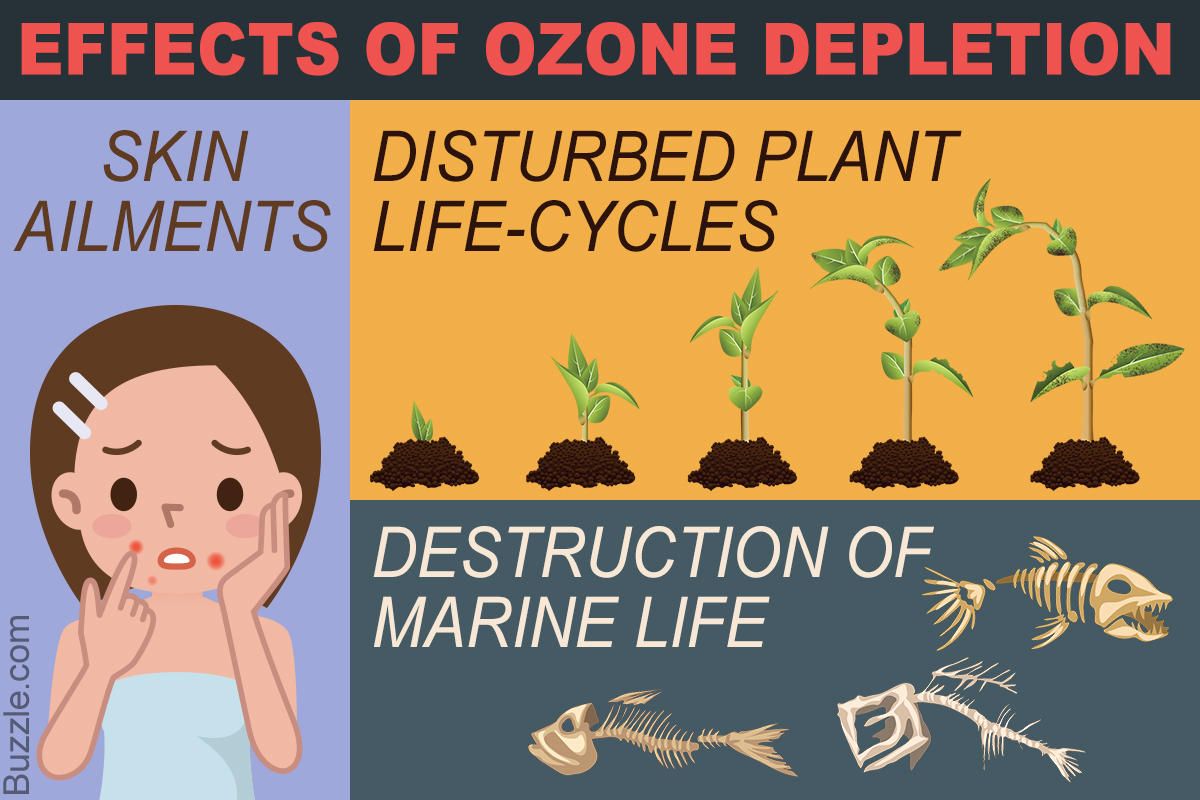
Effect of Ozone depletions
If the ozone is more depleted more ultraviolet radiation will reach the Earth’s surface.
Effect on plants:Will affect crop yield and forest productivity.
Effect on animals:Will cause damage to fish larvae and other small animals.
Effect on human health:It results in non-melanoma skin cancer and melanoma, acute erythemia,occur abnormalities, cataract and poor immune responses.
How can we prevent depletion of ozone?
⭐Avoid the consumption of gases dangerous to the ozone layer, due to their content or manufacturing process.
⭐Serving of refrigerators and air conditioners Should be regulated and minimize the use of cars.
⭐ Production,use and emission of ozone depleting chemicals should be controlled.
⭐ Refrigerators should be recaptured and used.
⭐Adopt protein measures from sun’s radiation.




You must be logged in to post a comment.