Prof Shankar Chatterjee, Hyderabad
Respected sirs, this is Professor Shankar Chatterjee, retired from an academic organisation of the Government of India. I am a senior citizen, have travelled widely in India and abroad for educational activities, and have published many books and research articles. I am proud of my country, as many foreigners—from Europe to South America, including Asian and African countries —have appreciated India for its development in science and technology.
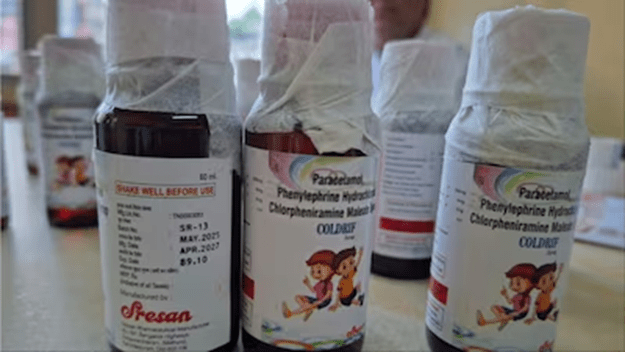
It is miserable, sad, and deplorable news that many babies died because of cough syrup. ‘Coldrif’ cough syrup has come to the spotlight after many children were killed in Madhya Pradesh and Rajasthan. A batch of the medicine collected from a Tamil Nadu manufacturing unit was found to contain the toxic chemical diethylene glycol (DEG) at levels exceeding permissible limits. The World Health Organisation (WHO) has repeatedly warned of cough syrups contaminated with DEG and ethylene glycol (EG), linking them to over 300 child deaths globally since 2022.
I have observed on television that the cough syrup company in Tamil Nadu is in a dilapidated, unhygienic condition. Anyway, the deaths are very unfortunate because the innocent babies will not come back to their parents due to the callousness/corruption of a pharmaceutical company. Through this publication, I request the Hon’ble Prime Minister and the Hon’ble Minister of Health & Family Welfare and Chemicals & Fertilizers to conduct a thorough survey of the pharmaceutical companies in India that produce different types of medicines. And the companies that do not adhere to rules and regulations should be closed. Because of earning profit through corrupt ways, innocents should not die. The good news is that India’s central drug regulator, the Central Drugs Standard Control Organisation (CDSCO), has started risk-based inspections at drug manufacturing units in six states after 19 samples of medicines—including cough syrups, antipyretics, and antibiotics—were collected for testing following child deaths in Madhya Pradesh and Rajasthan allegedly linked to contaminated cough syrups. The inspections, which began recently, aim to identify gaps that may have led to lapses in drug quality and to recommend process improvements to prevent such incidents in the future. The manufacturing units under scrutiny are located in Himachal Pradesh, Uttarakhand, Gujarat, Tamil Nadu, Madhya Pradesh, and Maharashtra. This pharmaceutical company should be prosecuted so that others cannot do such a notorious activity. And nationwide, a survey should be conducted on the pharmaceutical companies that produce cough syrup. We know medicine is meant to heal, not harm.


You must be logged in to post a comment.