COMPANY COMPLIANCES DURING THE COVID-19 ERA: AN INTRODUCTION

The global outbreak of the novel coronavirus has taken the world by storm. While the issue pertaining to the public health is the talk of the town, the impact of COVID-19 on businesses and corporates seems to be least talked about.
Day to day business of the corporates is being affected due to decreased inflow of the human resource and a decrease in the workflow. While technologies have provided a relief to the human resource for physical attendances and conferences, there seemed to be unsettled trouble regarding legal compliances that required various filings and physical meetings.
Pursuant to the ongoing global COVID-19 pandemic and the Finance Minister, Ms. Nirmala Sitharaman’s announcements on March 24, 2020, the Ministry of Corporate Affairs (“MCA”) has issued various circulars to provide relief to companies from certain compliances under the Companies Act, 2013 (“Act”) and associated rules. This has been done as a measure to reduce the compliance burden on entities during the unprecedented health and economic situation caused by COVID-19. Following are the measures:-
1. Company Affirmation of Readiness towards COVID-19
Social distancing has gained its importance as a way to contain the spread, morbidity, and mortality of COVID-19. Government of India (“GOI”), responsible for the public welfare at large, has realised that social distancing can be achieved in its true sense only if the employers of the Indian public make the same application in their respective premises.
Considering that major employers of the nation belong to the companies or limited liability partnership (“LLP”) type entity, GOI as part of disaster management have advised all companies/LLPs to put in place an immediate plan to implement the “work from home” policy as a temporary measure up till March 31, 2020.
Further, in case of a requirement of physical visits of the essential staff to such offices by the employers, staggered timings may be followed in order to minimize physical interactions of all kinds.
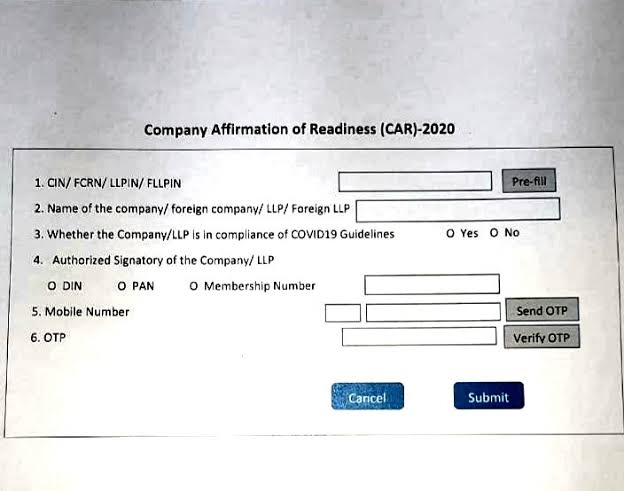
A simple webform for companies/LLP shall be deployed by MCA on March 23, 2020, in order to confirm the readiness of the employers to deal with COVID-19 threat. The webform shall be called CAR (Company Affirmation of Readiness towards COVID-19) and would be required to be signed and submitted by the authorised signatory of the company/LLP.
Therefore, it shall be expected by each company/LLP to ensure reporting of the compliance through CAR instantly from the date of its deployment.
2. Companies Fresh Start Scheme 2020

The MCA issued a circular on March 30, 2020, introducing the Companies Fresh Start Scheme, 2020 which, inter alia, grants a one-time opportunity to defaulting companies to complete all belated filings, including, without limitation, annual filings and filings required under IEPFA (Accounting, Audit, Transfer and Refund) Rules, 2016 in relation to transfer of money remaining unpaid or unclaimed for a period of seven years under Section 124(5) of the Act and transfer of relevant shares in the name of the ‘Investor Education and Protection Fund’ under Section 124(6) of the Act, with the MCA21 registry, without incurring additional fees on account of any delay.
This scheme came into force on April 1, 2020, and is valid till September 30, 2020. The application for seeking immunity for belated filings under this scheme should be made within a period of six months from September 30, 2020, through Form CFSS-2020. Thereafter, an immunity certificate will be provided by the designated authority on the basis of the declarations made in such form.
However, no immunity shall be provided under the scheme in a matter where (i) an appeal or management dispute is pending before any court or tribunal, or (ii) a court has ordered a conviction, or the adjudicating authority under the Act has imposed a penalty, and in respect of such orders, no appeal has been filed prior to the scheme coming into force.
Further, the scheme shall not apply: (i) where an application has been filed or an action for final notice for striking off the name of the company has already been initiated; (ii) where the company has been amalgamated; (iii) when application of obtaining dormant status has been filed; (iv) to vanishing companies; and/or (v) where charge related documents or an increase in authorised capital is involved.
3. CSR Spending

The MCA has by way of circular dated March 23, 2020 and the office memorandum dated March 28, 2020, clarified that the spending of CSR funds by companies in relation to COVID-19, including by way of contribution to the PM CARES Fund, is an eligible CSR expenditure under the Act.
The MCA has further clarified by way of FAQs dated April 10, 2020 that contributions made to the State Disaster Management Authority will also be eligible CSR activity, but contributions towards (a) ‘Chief Minister’s Relief Fund’ or ‘State Relief Fund for COVID-19’; and (b) payment of salary/ wages to employees and workers (including contract labour/ temporary/ casual/ daily wage workers) during the lockdown period will not be considered as eligible CSR expenditure.
However, ex-gratia payment over and above the disbursement of wages to temporary/ casual workers/ daily wage workers, specifically for the purpose of fighting COVID-19, will be admissible towards CSR expenditure, provided there is an explicit declaration to that effect by the board of the company, which is duly certified by the statutory auditor.
4. Meetings of Board and the Shareholders

- The Companies (Meetings of Board and its Powers) Rules, 2014 were amended by a notification dated March 19, 2020, to enable companies to hold board meetings on the following matters (which earlier had to be necessarily held at a physical meeting) through video-conferencing or other audio-visual means (collectively “VCC”) till June 30, 2020: (i) approval of annual financial statements and board’s report; (ii) approval of prospectus; (iii) audit committee meetings for consideration of financial statements; and (iv) approval of amalgamation, merger, demerger, acquisition and takeover.
- MCA has, by way of a general circular dated April 8, 2020, requested companies to pass all decisions of an urgent nature requiring shareholder approval, other than those of ordinary business or business where any person has right to be heard, through postal ballot/ e-voting in accordance with the relevant statutory provisions without holding a physical general meeting. However, in cases where holding an extraordinary general meeting (“EEGM”) is unavoidable, these have now been permitted to be held through VC until June 30, 2020. The circular further lays down certain conditions to be met for conducting an EGM through VC and the key conditions, inter alia, include: (i) attendance of at least one independent director (where a company is required to appoint one) and auditor (or his authorised representative who is qualified to be the auditor); (ii) maintenance of recorded transcripts of the EGM and, in case of a public company, such transcripts to be uploaded on the company website (if any); and (iii) e-voting facility being available. All other provisions relating to general meetings under the Act (and relevant rules) will continue to apply.
- Due to difficulties faced by various stakeholders in serving and receiving notices/responses by post on account of COVID-19, the MCA, on April 13, 2020, provided that notice of EGMs to be held through VC (and for passing shareholder resolutions through postal ballot/ e-voting) may now be given to shareholders only through email addresses of the shareholders registered with the company or with the depository participant/ depository. This circular also specifies various conditions which companies must comply with while sending email notices to shareholders.
CONCLUSION
Business entities in India are requested and expected to keep an eye on the major government websites to ensure timely compliance with all such immediate requirements and mandates issued by GOI as need of the hour from time to time.
WEBSITES REFERRED:-
1) MCA General Circular No. 10/20 dated March 23, 2020 on Clarification on spending of CSR for COVID-19.
2) MCA General Circular No. 12/20 dated March 30, 2020 on Companies Fresh Start Scheme, 2020
3) MCA Notification dated March 19, 2020 on Companies (Meetings of Board and its Powers) Amendment Rules, 2020
4) MCA General Circular No. 14/2020 dated April 8, 2020 on Clarification on passing of ordinary or special resolutions by companies under the Companies Act, 2013 and rules made thereunder on account of threat posed by Covid-19.
5) MCA General Circular No. 17/20 dated April 13, 2020 on clarification on passing ordinary and special resolutions by companies under the Companies Act, 2013 and rules made thereunder on account of threat posed by COVID-19.
6)http://www.conventuslaw.com/report/india-implications-of-covid-19-on-compliances/
7)https://www.lexology.com/library/detail.aspx?g=7862d71f-35ae-443c-964b-a381d11102bc
8)https://www.google.com/search?q=COMPANY+COMPLIANCE+India+Images+Copyright+Free+and+Royalty+Free&tbm=isch&ved=2ahUKEwjK6fe59tvqAhVZOCsKHTZKCh0Q2-cCegQIABAC&oq=COMPANY+COMPLIANCE+India+Images+Copyright+Free+and+Royalty+Free&gs_lcp=ChJtb2JpbGUtZ3dzLXdpei1pbWcQAzoECB4QCjoECCEQClCMPljJiQFgoIwBaARwAHgAgAHIAYgB4x2SAQYwLjI3LjGYAQCgAQHAAQE&sclient=mobile-gws-wiz-img&ei=qpoVX8rsBdnwrAG2lKnoAQ&bih=682&biw=393&client=ms-android-xiaomi-rev1&prmd=insv#imgrc=UEkUjY7KpsptxM
9)https://studycafe.in/2020/04/companies-fresh-start-scheme-2020-or-cfss-2020.html
10)https://www.a2ztaxcorp.com/mca-introduces-companies-fresh-start-scheme-2020-for-non-compliant-companies/
11)https://www.istockphoto.com/illustrations/corporate-social-responsibility?mediatype=illustration&phrase=corporate%20social%20responsibility&sort=mostpopular
12)https://www.istockphoto.com/illustrations/shareholders-meeting?mediatype=illustration&phrase=shareholder%27s%20meeting&sort=mostpopular













You must be logged in to post a comment.