World Ozone Day 2022 falls on
September 16 to commemorate the signing of the Montreal Protocol, an
international treaty to protect the ozone layer by phasing out the production
of many substances.
World Ozone Day 2022 is
celebrated around the world on September 16 every year to start a conversation
and raise awareness about the depletion of the ozone layer. Each year, the
United Nations announces a theme for World Ozone Day, focusing on immediate
action that must be taken by people and governments. Ozone is a protective
layer in the Earth’s atmosphere that absorbs most of the ultraviolet radiation
that reaches Earth from the Sun. World Ozone Day 2022 is celebrated to spread
awareness about ways that are effective in protecting the ozone layer.
Date of World Ozone Day 2022
World Ozone Day is celebrated
every year on September 16 to highlight and spread awareness about the
importance of the ozone layer in protecting the Earth.
Theme of World Ozone Day 2022
The theme of World Ozone Day 2022
is “Global Cooperation to Protect Life on Earth”. The theme of World
Ozone Day, proclaimed by the United Nations, emphasizes the collective efforts
that must be made to protect life on Earth.
History of World Ozone Day 2022
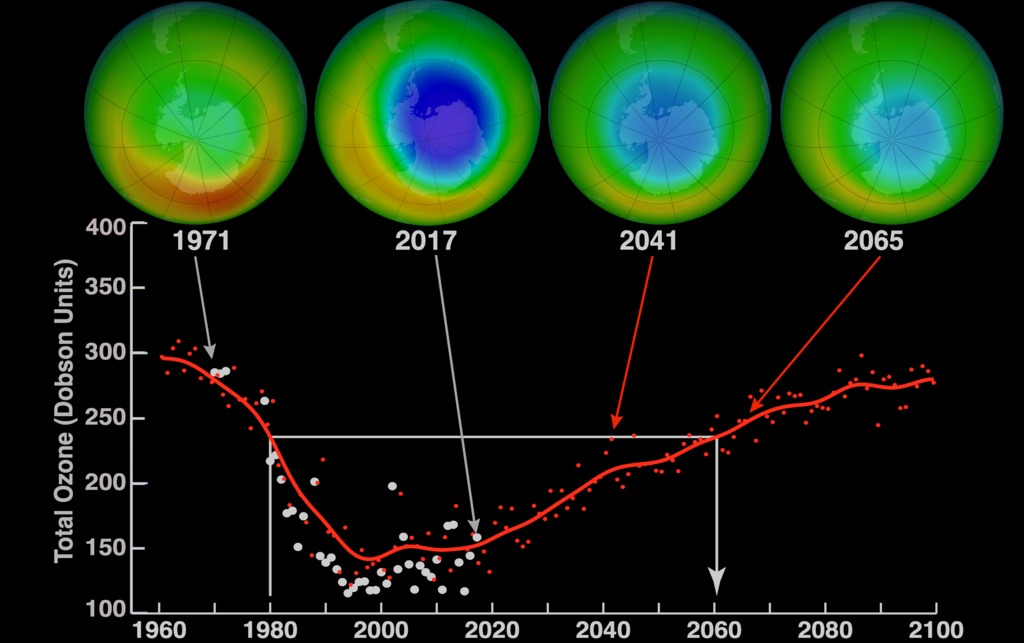
World Ozone Day was first
observed in 1995 and was celebrated to raise awareness of the importance of the
ozone layer to the environment. When scientists discovered in the 1970s that
humanity was making a hole in the ozone layer, they expressed their concerns.
Based on it, the governments of
the whole world adopted the Vienna Convention on the Protection of the Ozone
Layer in 1985 and decided to save it.
World Ozone Day on 16 September
commemorates this achievement and shows that collective decisions and action
are the only way to solve major global crises led by science.
World Ozone Day 2022: Montreal
Protocol?
The main objective of the
Montreal Protocol is to protect the ozone layer by taking measures to control
the total worldwide production and consumption of substances that damage it,
with the ultimate goal of eliminating them based on the development of
scientific knowledge and technological information. Groups of chemicals are
classified by chemical group and are listed in annexes to the text of the
Montreal Protocol. The protocol calls for the control of nearly 100 chemicals
in several categories. For each group or annex of chemical substances, the
Agreement establishes a schedule for the gradual cessation of production and
consumption of these substances, with the aim of eventually eliminating them
completely.
The schedule established by the
Protocol applies to the consumption of ozone-depleting substances. Consumption
is defined as the amount produced plus imported minus the amount exported in a
given year. There is also a deduction for certified destruction. Percentage
reductions refer to the specified baseline year for the substance. The Protocol
does not prohibit the use of existing or recycled controlled substances after
the phase-out date.
There are a few exceptions for
essential uses where no acceptable substitutes have been found, such as in
metered dose inhalers (MDIs) commonly used to treat asthma and other
respiratory problems, or in halon fire extinguishing systems used in submarines
and aircraft.
In 1994, the United Nations
General Assembly declared 16 September the International Day for the
Preservation of the Ozone Layer, commemorating the signing of the Montreal
Protocol on Substances that Deplete the Ozone Layer in 1987 (resolution
49/114).




You must be logged in to post a comment.