Nagarale, D. V., Khairnar, B. J., & Girase, P. S. (2026). Antimicrobial Investigation of Knoevenagel Products of Quinoline Derivatives. International Journal of Research, 13(13), 68–73. https://doi.org/10.26643/ijr/2026/s13/7
1Deepak Vasant Nagarale, 2Bhikan J. Khairnar, 3Pravinsing S. Girase*
1*Department of Chemistry, VVM’s S. G. Patil College, Sakri, Dhule
2S. S. V. P. S’s. L. K. Dr. P. R. Ghogrey Science College, Dhule
3M. D. Sisode College, Nardhana
Corresponding Authors Email: deepaknagarale03@gmail.com
Abstract:
We have described anti-microbial screening of synthesized compounds. All these compounds screened giant’s standard antibacterial and antifungal agents. The detail of biological activity of these active compounds were discussed and results are moderate to high the zone of inhibition obtained.
Kay Words: Antibacterial, Antifungal agents, dicarbaldehydes, Microorganism.
Introduction:-
Involvement in biological procedures makes particular molecular unit uniquely important and interesting [1]. However, the very importance of biological processes has fostered piecemeal approaches to the description of functional relationships between biological activities and the chemical substances that express them. The biological activity of a organic compound is given by A=cf, where A is the activity, c is the concentration of substance and f is a parameter designated as ‘‘inherent activity.’’ The type of activity, catalytic (katal) or binding (mol-1 L) determines units and dimensions[2].Biological activity provides both biologically information and that enables development of the quantitative dimensions needed to exploit the new knowledge [3].
8.2 Biological Activities:-
In this paper we have described anti-microbial screening of synthesized compounds. All these compounds screened giants standard antibacterial and antifungal agents. The detail of biological activity of these active compounds were discussed and the results are given in TablesThe evaluation done for various derivatives for antibacterial activity and literature procedure shows agar diffusion method by finding the zone of inhibition of the drug sample against the standard drugs.
Procedure-I: Stock solutions of test compounds and standard drug:-
Compounds were taken as test samples along with a standard drug samples. 10 mg of each test compound dissolved in 1 ml of DMSO for preparing stock solution of standard drugs.
Organisms used for experiment:-
The organisms employed in the in vitro testing of the compounds were Staphylococcus aureus (Gram positive), Bacillus subtilies (Gram positive), Pseudomonas aeruginosa (gram negative), Escherichia coli (gram-negative) bacterias and Candida albicans (gram-negative) fungi. All the cultures maintained on nutrient agar (Microbiology grade, Hi Media) medium by periodic sub culturing.
Preparation of inoculum:
Procedure for the preparation of inoculums for all the organisms was same. The inoculum was prepared within 24 hrs. From old growth of organism on nutrient agar slant with the help of sterile nichrome wire loop. The growth of the organism on slant especially transferred to a tube containing sterile distilled water.
Medium:
Nutrient agar (1.5 g, Microbiology grade, Hi Media) dissolved in sterile distilled water (100 ml) and Poloxomer 182 (3 g)added as a surfactant to the media to prevent the drug precipitation. 20 ml of this stock solution taken to each Petri dish.
Test Mechanism:-
Petri dish containing approximately 20 ml of disinfected nutrient agar, 0.1 ml of reliable culture of test organisms spread. The stock solution added from four bore wells having 5-20 µl[4,5].This matches to concentration range of 30 µg/ml of the test compound. The tests carried out in duplicate. To one side from placing the controls of standard drug (Ciprofloxacin), controls with dimethyl sulphoxide (Positive control) and without dimethyl sulphoxide (Negative control) were also contained within in the test[6].
Incubation:-
Incubation period for microorganism growth was 37°C for 24 hours under dark conditions. Zone of inhibition were determined at the end of incubation period [7].
8.3 Results and Discussion:-
Section-I: Antimicrobial Study of Knoevenagel Products of Quinoline:-
We have synthesized biologically active Knoevenagel products of 2-chloro-3-formyl quinolines with active methylene compounds, which shown in Tableand results of their microbial screening shown in table.
Table 1: Synthesized Knoevenagel Products of Quinoline
| | Sr. No. | Compound No. | R- |
| 3a | -H | ||
| 3b | -H | ||
| 3c | -H | ||
| 3d | -H | ||
| 3e | -H | ||
| 3f | 6-Cl, 8-NO2 | ||
| 3g | 6-Cl, 8-NO2 | ||
| 3h | 6-Me | ||
| 3i | 6-Me | ||
| 3j | 6-Me | ||
| 3k | 6-Cl | ||
| 3l | 6-Cl | ||
| 3m | 6-Cl | ||
| 3n | 7-OMe | ||
| 3o | 7-OMe | ||
| 3p | 7-OMe | ||
| 3q | 7-OMe | ||
| 3r | 7-OMe |
Table 2: Antimicrobial Screening of Knoevenagel Products of Quinoline
| Compound No. | Inhibition Zone Diameter (mm) | |||||
| Sample code | S.aureus | B.subtilis | P. aureginosa | E. coli | C. albicans | |
| 3a | Pm33 | – | – | – | – | 8.24 |
| 3b | Pm34 | 7.34 | 7.12 | – | – | 7.53 |
| 3c | Pm35 | – | – | – | – | – |
| 3d | Pm36 | – | – | – | – | – |
| 3e | Pm37 | – | – | – | – | – |
| 3f | Pm38 | 10.26 | 10.58 | – | – | 11.28 |
| 3g | Pm39 | – | – | – | – | 10.60 |
| 3h | Pm40 | – | – | – | – | 6.90 |
| 3i | Pm41 | – | – | – | – | 7.10 |
| 3j | Pm42 | – | – | – | – | – |
| 3k | Pm43 | – | – | – | – | 9.27 |
| 3l | Pm44 | – | – | – | – | 8.96 |
| 3m | Pm46 | – | – | – | – | – |
| 3n | Pm47 | – | – | – | – | – |
| 3o | Pm48 | – | – | – | – | – |
| 3p | Pm49 | – | – | – | – | – |
| 3q | Pm50 | – | – | – | – | – |
| 3r | Pm51 | – | – | – | – | – |
| Chloramphenicol | – | 24.58 | 24.55 | 25.22 | 25.44 | – |
| Amphotericin B | – | – | – | – | – | 12.58 |
| “ – ” Zone of Inhibition | ||||||
Figure 1: Line graph of Antimicrobial Screening of Knoevenagel Products of Quinoline
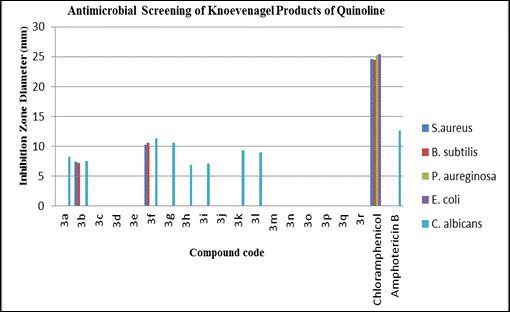
Antibacterial Study:-
Compounds 3b, 3f showed moderate activity against S. aureus, and B.subtilisr respectively when compared to the standard drug Chloramphenicol at tested concentration.
Antifungal Study:-
Among the screened compounds 3f, 3g showed significant and 3a,3b, 3h, 3i, 3k and 3l showed moderate activity against C. albicans when compared to the standard drug Amphotericin B at tested concentration.
Conclusion:-
We conclude the overall observation about some of the synthesized compounds. Compounds were showed antimicrobial activity, with compared to standard drugs. Compounds 3b, 3f,5a, 5b, 5d, 5e have showed moderate anti Bacterial activity and compounds 3f, 3g, 5b and 5d have showed superior antifungal activity, while 3a, 3b, 3h, 3i, 3k, 3l, 5c, 5e have showed moderate antifungal activity.
References:-
- World Health Organization(2000) ,WHO consultation on international biological standards for in vitro diagnostic procedures. WHO, September (2000), Geneva, Switzerland.
- C. M. Jackson, M. P.Esnouf, D. J. Winzor, D.L. Duewer, A.Q.Assur, 12(2007), 283.
- J. Wyman., J.M.Biol., 11(1965), 631.
- G.A.Pankey and L.D.Sabath.,Clin. Infect, Dis, 38(2004), 864.
- A. E.Zoerby, F.Sanschagrin,R. C. Levesque.,Mol. Microbiology, 47(1) (2003),1.
- W.Foye. Principles of Medicinal Chemistry (IVthEdn.), WAVERLY,821.
- T. W. Chu Daniel, J. J. Plattner., J. Med. Chem, 39(20),(1996), 3853.


You must be logged in to post a comment.