Citation
Pawar, H. S., & Nandre, S. J. (2026). Comparison of Growth and Characteristic Properties of Barium Oxalate and Cobalt Oxalate Single Crystals. International Journal of Research, 13(13), 101–107. https://doi.org/10.26643/ijr/2026/s13/10
Comparison of Growth and Characteristic Properties of Barium Oxalate and Cobalt Oxalate Single Crystals
H. S. Pawar1, S. J. Nandre2
1V.J.N.T. Late Dalpatbhau Rathod Arts and Science College, Mordadtanda (Dhule) M.S
Email ID: pawar.hs1188@gmail.com
2Department of Physics, Uttamrao Patil Arts and Science College, Dahiwel (Dhule) M.S
Abstract:
The barium oxalate and cobalt oxalate single crystals were grown in agar-agar using gel method. Then compared the growth parameters. The XRD, FTIR, TGA/DTA analysis of the grown crystals confirmed the crystals crystalline. Morphology of the crystals studied from the photography while the structures of the crystals were studied from XRD. Thermal analysis reveals the decomposing temperature of the crystals.
Keywords: Barium oxalate, Crystal growth and Cobalt oxalate.
Introduction
Crystal growth is the basis of numerous technology improvements. The gel growth method is the most efficient and simple process of crystal growth. It is considered more useful at ambient temperature and has an inexpensive process. The contribution of gel method in crystallization has been significant. This method has been successfully developed as one promising way of growing single crystals and gives many interesting results, from simple metals salts to complex compounds. Single crystals are the backbone of the modern technology of logical revolution [1-3]. The impact of single crystal, is clearly visible in industries like semiconductors, optics etc. Growth and characterization of oxalate single crystals have attracted many researchers single crystals of oxalate single crystal have been grown and reported. Now a day great attention has been devoted on the growth and characterization of oxalate crystal with the aim of identifying new materials for practical purposes. It is well established that there are extensive study on oxalate based crystal grown by gel technique; however, we have found that there are few reports on the barium and cobalt oxalate based crystal because of their chemical properties. Therefore, in the present study, we have investigated the growth mechanism of barium oxalate and cobalt oxalate crystals. All both types of crystals were grown by gel method by using single diffusion techniques [4].
Experimental
In the present work, barium oxalate and cobalt oxalate single crystals were grown by single diffusion technique. The growth of barium oxalate crystals was carried out in agar-agar gel by adopting the similar technique as reported (Dalal and Saraf 2009) [6]. Barium chloride (BaCl2, 99.9%), Cobalt chloride (CoCl2, 99.9%) Oxalic acid (H2C2O4, 99%), Agar-Agar powder (C14 H24 O9) were used as the starting materials. All chemicals were AR grade. The borosilicate glass tube was used as crystallization apparatus. The glass tubes used for single diffusion were of 25cm length and 2.5cm outer diameter. The solution of different (0.5, 1.0, 1.2, 1.5, 2M) were prepared and store in clean glassware. Agar-Agar gel was prepared by mixing (0.5 to 2.0gm) of agar powder in 100ml double distilled water at boiling temperature. Barium chloride and Cobalt chloride of concentration 0.5 to 2 M and oxalic acid of concentration 0.5 to 2M were used as reactants [5]. The prepared solution of oxalic acid were then transfer into the test tube, after that appropriate volume of agar gel poured in this test tube and the mouth of test tube closed by the cotton plug and then kept undisturbed for aging period of few days. Usually with 24 to 36 hours the gel was found to set which depends on the environmental temperature. Room temperature and atmospheric effect also plays an important role on gelation, aging that is evaporation of water molecules from on surface of gel. It was observed that the mixture in glass tube was initially transparent and slowly turn milky white. After insuring firm gel setting it was kept for aging for 2 to 3 days. Aging makes the gel harder and reduces the diameter of capillaries present in the gel. After setting and aging over the set gel solution of second reactant (supernatants) of desire volume and molarity was poured gently along the wall and allowed to diffuse into the gel medium. The open end of test tube was closed with cotton plug to prevent evaporation and contamination of the exposed surface by dust particles and impurities of atmosphere and was kept undisturbed [6].The following chemical reactions were employed for the growth
BaCl2 + H2C2O4 BaC2O4 + 2HCl……(Barium Oxalate)
CoCl2 + H2C2O4 CoC2O4 + 2HCl….(Cobalt Oxalate)
Results and Discussions
Fig.1 a) shows Lieseggang rings and the grown crystals, b) shows working reaction during crystal growth in test tube. c) shows Harvested crystals of cobalt oxalate and d) barium oxalate single crystals
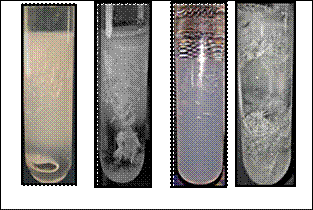

Figure 1 a) Figure 1 b)
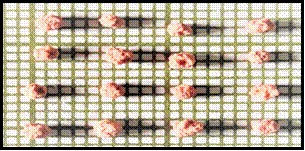

Figure 1 c) Figure 1 d)
The characterization of the pure and doped crystals were carried using XRD, FTIR, thermal analysis, UV absorption spectrum and scanning electron micrographs and their characteristics are compared.
X-Ray diffraction: The X-ray diffraction pattern of barium oxalate and cobalt oxalate crystals are shown in Fig. 2. The patterns of these two samples were taken at room temperature in order to study the structure of the materials. Both materials were found to be single crystalline. From the XRD pattern it is noticed that the peaks obtained at 23.83, 29.44, 39.37, 44.79, 46.71, and 59.020 are corresponds to the (002), (102), (301), (020), (120) and (-104) lattice planes of the barium oxalate crystals and for the cobalt oxalate the peaks are observed at 18.75, 30.23, 34.96, 43.28,51.19, 56.09 and 60.730 are corresponding to the (111), (220), (311), (021), (314), (422) and (511) lattice planes [3].The lattice parameters of barium oxalate a= 8.2425Å, b = 4.0457Å and c = 6.4707 Å, volume of unit cell, V = 215.61 Å3 and of cobalt oxalate a = 5.39820 Å, b = 5.03100 Å, c = 5.73590Å, volume of unit cell, V=155.77 Å3. From the calculated (hkl) and ‘d’ values, it is found that the both oxalate crystals crystalize in the monoclinic system. Table 1. Comparison of Lattice parameters.
FTIR: Thermo- Nicolet, Avatar 370 spectrophotometer is used for the study of FTIR spectrum of both samples. KBr is used as the beam splitter and also as detector. The peaks are identified in comparison with earlier reports. The broad peak at 3600-3400 cm-1 due to anti symmetric O-H
stretching suggest the presence of water of crystallization in both crystals. All the functional groups are observed in both types of crystals and obtained data is summarized in Table 2 [7].
Table 1. Comparison of Lattice parameters
| Material | Chemical formula | System | Lattice parameters a, b, c, α, β, γ | Volume (Å)3 |
| Barium Oxalate | BaC2O4 | Monoclinic | a = 8.2425Å b = 4.0457Å c = 6.4707 Å | 215.61 Å3 |
| Cobalt Oxalate | CoC2O4 | Monoclinic | a = 5.39820 Å b = 5.03100 Å c = 5.73590Å | 155.77 Å3 |
Table 2 Summary of vibrational infrared frequencies of different bonds found in the grown crystals
| Fundamental frequencies | Barium oxalate | Cobalt oxalate |
| O-H Stretching and water of crystallization | 3566 | 3366 |
| Metal oxygen bonding | 590 | 487 |
| C = O stretching | 51652 | 1621 |
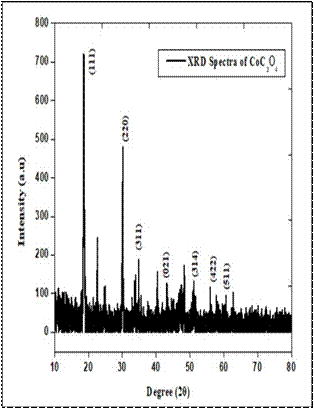
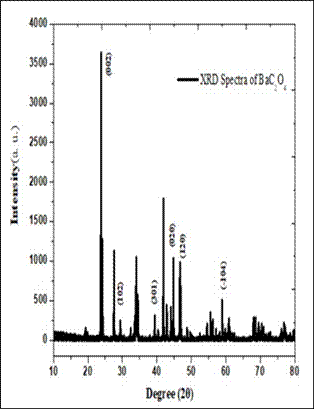
Fig. 2 X-ray diffraction pattern of a) Cobalt oxalate b) barium Oxalate
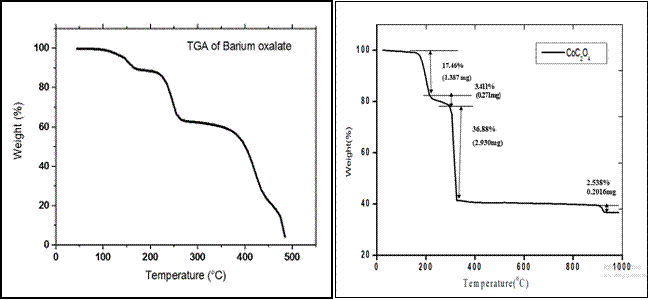
| Fig. 4.13 TGA of Barium oxalate crystal grown by agar-agar gel |
Fig. 3 TGA of Barium oxalate and cobalt oxalate crystal grown by agar-agar gel
| Fig. 5.13 TGA of Cobalt oxalate crystal grown by agar-agar gel |
From the TG diagram, barium oxalate crystal showed four stages of weight loss. Thus the curve shows a gradual mass loss. From this graph, the weight loss starts at around 500C and steps at 172oC in which weight loss of 5.17%. It is observed that the material is stable up to 50oC. The second stage of decomposition is from 1720C to 277oC in which weight loss of 25.68%. In the third stage of decomposition is from 2770C to 4350C in which weight loss of 36.68%. In last stage of decomposition is from 4350C to 4780C in which weight loss 13.45%. The residue at the end is at 4780C. The TG thermo gram cobalt oxalate reveals that decomposition starts at 300C and steps at 1770C in which weight loss 17.46%. In the second stage of decomposition in the temperature range 2000C to 2470C, the total weight loss 3.411%. In the third stage of decomposition total weight loss 36.88% was observed in the temperature range 2470C to 2600C and last stage in the temperature range 8920C to 9300C total weight loss of 2.538% was obtained. The residue at the end is at 9300C. [8-11]
Acknowledgment
The author are grateful to research guide Dr. S. J. Nandre, Uttamro Patil Arts and Science college, Dahiwel (Dhule) M.S. also thankful to Prof. Mukesh Padvi, Department of Physics, Shivaji University, Kolhapur and Mr. Rushikesh P. Dhavale, Department of material Science and Engineering,Yensei University, Seoul Republic of Korea for providing characterization facilities.
References
- P. V. Dalal and K. B. Saraf, “Bulletin of Material Science”, vol-29, pp. 421-425, (2006).
- P. V. Dalal, “Indian Streams Research Journal”, vol-5, pp1-4, (2015).
- P. V. Dalal, K. B. Saraf and S. shah, “Crystal Research and Crystals Technology”, vol-44, pp36-42, (2009).
- M. R. Shedam, M. S. Rakesh and N. M. Shridhar, “Journal of Nano-Electron Phys.”, vol-8, pp-04075, (2016)
- P. V. Dalal, “Indian Streams Research Journal”, vol-5, pp1-4, (2015).
- P. V. Dalal, K. B. Saraf and S. shah, “Crystal Research and Crystals Technology”, vol-44, pp36-42, (2009).
- Dewei Wang et. al., “Inorganic chemistry”, vol-50, pp6482-6492, (2011).
- Anilkumar Kodge et. al., “International Jour. of Eng. Sci. and Tech.”, vol-3, pp6381-6390, (2011).
- E. Romero et. al., “Chines Science bulletin”, vol-48, pp1844-1852, (2003).
- S.J.Nandre, S.J.Shitole, S.S.Sonawane and R.R.Ahire, “International Journal of Basic and Applied Research”, vol-4, pp125-128, (2012).
- G. A. Kumar, “J. Phys. Chem. Solids”, vol-62,pp-1327, (2001).


You must be logged in to post a comment.