M. A. Patil1 G.H. Sonawane1
Kisan Arts, Commerce and Science College, Parola Dist Jalgaon (M.S.), India.
Abstract:-The intriguing features of MXene, a novel family of two-dimensional materials, include strong surface area, negative zeta potential, metallic conductivity, and electric conductivity.The majority of Mxene are currently only successfully prepared by exfoliating MAX with high concentration hydrofluoric acids. In this study, the 2D Ti3C2 with large interplanar spacing was successfully achieved by alkali mixture of NaF and HCl, in single process. The morphology and structure of prepared sample characterized by XRD and SEM. This work presents a safely effective route to synthesize the 2D Ti3C2. Fabrication of ZnO/MXene composites by a facile chemical method. Under UV irradiation, Rhodamine B was degraded by composites within 15 min and retained photo-catalytical efficiency after 5 cycles. Therefore ZnO/MXene composites can be regarded as aeffective candidate for waste water treatment and environmental protection.
Keyword:- MAX phases, MXene, ZnO, Rhodamine B
1.Introduction:-Due to the discovery graphene in 2004[1-3], The 2D materials have attracted researcher interest. Owing to the reduction of the dimension and size, two-dimension materials have exhibited many intriguing properties that are not found in their bulk counters, holding tremendous promise for a host of applications ranging from electronics[4-6] and optoelectronic device[7, 8], photocatalysis[9, 10]to electrochemical catalysis[11, 12]. In recent years, with great advances in the synthetic techniques, more 2D materials beyond graphene have been successfully produced such as silicene[12], silica glass[13], molybdenum disulfide[14, 15], germanene[16, 17], stantene[18], phosphorene[19]. Among the, a newly discovered large family of 2D large family of transitional metal carbide/ nitride or carbonitride called ‘’MXene’[20], is rapidly rising starThese novel materials are produced from MAX phaseswith selective remove A layered using etchants without destroying M-X bond because the M-X bonds are much stronger than the M-A bonds[21]. MAX phasesare layered ternary compound with general formula of Mn+1AXn(n=1,2,3), where M represents early transition d block transition elements, A is predominately IIIA or IV A group element, and X is either C or N,MAX phases possess hexagonal layered structure in which Mn+1Xn units and A layers are alternatively stacked.After the exfoliation resulting surface of MXene are terminated with other groups, such as -F, -OH and -O[22]. So, the MXene represents as Mn+1XnTx, Where T is the surface terminal groups depends upon etchants solution and condition. Experimentally, the proportions of different functional groups on the MXene surface are uncertain.In case of hydrothermal or electrochemical etching methods absence of terminal functional groups and represented as Mn+1Xn such as Ti3C2[23]. Most of MXeneshows metallic behavior exhibiting electronic conductivity higher than all other solution possessed 2D materials. These materials have shown significant promise in variety of applications including electrochemical energy storage[24], electromagnetic interference shielding[25], gas sensing[26], and many other. In particular, the good flexibility of MXene make easy to form composite with other materials, which provide an opportunity of integrating the outstanding properties of different materials in a complementary way. MXene also has exceptional capacity to transport photogenerated electron from closely coupled semiconductor photocatalyst and suppress the recombination of electron hole pairs[27]. However, MXene based photocatalyst system with more efficient for removal of water pollutants is still needed to develop.
ZnO is widely applied semiconductor photocatalyst in pollutants removal including heavy metal ions[28] and organic contaminants[29], and it has a strong oxidation capacity and a wide band gap (~3.3 ev)[30] because its valence band is sufficiently to generate hydroxy radicals[31]. On the other hand, ZnO shows fast recombination of electron hole pairs[32] and shows poorest photocatalytic degradation of dyes[33]. Based on above consideration, we constructed an efficient heterojunction photocatalyst for degradation of hazardous water pollutants which consisted of MXene sheets and ZnO. These heterojunctionsfacilitate minimize the photogenerated electron transfer distance. Moreover, the heterojunction structure between the stable ZnO and high conductive layered structure of Ti3C2Tx, MXene can further facilitate the separation and transfer capacities of photogenerated charge carriers. Therefore ZnO/Ti3C2Tx exhibited excellent photocatalyst. This work provides new insight into for development of traditional semiconductor photocatalyst for traditional semiconductor photocatalyst for highly efficient degradation of Rhodamine B as waste water pollutants.
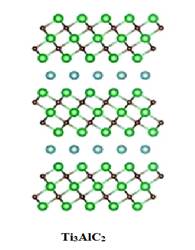
Fig 1.) Schematical representation of Crystal Structure of Ti3AlC2and monolayer of Ti3C2
2.Experimental Section
Etching Methods. Etching using NaF + HCl Solutions The etchant was prepared by adding 0.8 gm of NaF to 10 mL of 9M HCl and continuously stirring the resulting mixture for 10 min then 0.5 g ofTi3AlC2 powder gradually over the course of 5 min added into above etchants avoids excessive bubble formation of H2 gas, and resultant mixture were left under continuous stirring for 18 h at room temperature. Each reactant was centrifugation with DI water until pH~6.
Synthesis of ZnO/MXene composite 110 mg Zn(CH3COO)2.2H2O were dissolved into 50 ml of ethanol under vigorous stirring for 30 min at room temperature. Then 32 mg NaOH were dissolved into 50 mL of ethanol under vigorous stirring for 30 min, the two solutionswere mixed followed by addition of the 410 mL of ethanol. 0.25 gm of MXene was added in the solution under magnetic stirring at 600C for 40 min. The resulting precipitate was cooled down and sediment was collected by centrifugation. Finally, the precipitate was dried at 600C in autoclave for 18 h obtained as ZnO seeds/MXene
37.10 g Zn(NO3)2.6H2O andobtained ZnO/MXene were dissolved into 500 mL of DI Water in round bottom Flask,heated in oil bath at 1050C for 30 min. Then, 17.50g hexamethylenetetramine was added heated and stirred for 23h. Finally, after the reaction, process, the product was collected by centrifugation and dried in hot air oven at 600C for10 h
XRD of 2-D MXene:- The result revealed that the characteristics 002 peak located at 2θ=8.83A0In bare MXene, the 002 peak was found to be at 19A0 presenting as increase interlayer spacingthis peak not appear into bulk counter part of MAX phases
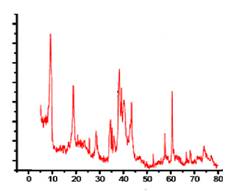
Fig 2) XRD image of Ti3C2 2-D Sheets
3. Photocatalytical Application of ZnO/MXene composite
The Photocatalytical performance were evaluated through removal of Rhodamine B as typical pollutants 200 mg ZnO/MXene composites were dispersed into 40 ml of 50 ppm solution of Rhodamine B, uniformed stirring with help of magnetic stirring. The solution kept in dark for 30 min and then irradiated withUV light for 18 min the reaction sample were collected at regular of 3 min for UV-visible analysis. The same set of experiment carried using 100 mg of ZnO particle were used to evaluated photocatalytical performance and the rhodamine b solution were collected at regular interval of 20 min for UV-Visible analysis
The degradation efficiency was evaluated by comparing percentage of degradation using following formula
η = (1-Ct/C0) where η is photodegradation in % and C and C0 are concentration of RhB solution after and before UV radiation, respectively. C/C0 calculated by A/A0, because the concentration of solution is directly proportion to absorbance of solution
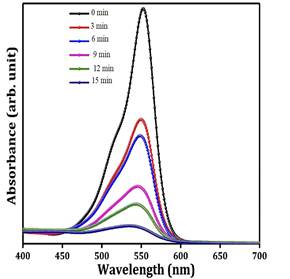
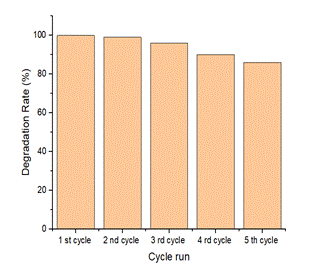
Fig 3a) Photo catalytical degradation of Rhodamine B under UV light3b) Photodegradation efficiency of ZnO/MXene upto 5th cycle runs
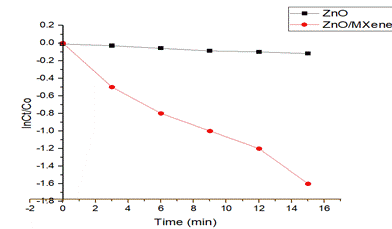
Fig 4) comparison of photocatalytic degradation of Rhodamine B using ZnO andZnO/MXene
4. Conclusion: –In summary, ZnO/MXene compositehave fabricated by two step facile chemical methods. The ZnO microrod/MXene composite within 15 min and more photocatalytical efficiency after 7cycles. ZnO/MXene composite is superior photocatalyst as compared to ZnO microrods. Therefore, the study opens new avenue for waste water pollutants and environmental protection.
5. Reference
1. Taghioskoui, M., Trends in graphene research. Materials today, 2009. 12(10): p. 34-37.
2. Kheirabadi, N., A. Shafiekhani, and M. Fathipour, Review on graphene spintronic, new land for discovery. Superlattices and Microstructures, 2014. 74: p. 123-145.
3. Kumar, C.V. and A. Pattammattel, Discovery of graphene and beyond. Kumar CV, Pattammattel A (Eds.,) Introduction to Graphene: chemical and biochemical applications, 2017: p. 1-15.
4. Han, T.-H., et al., Graphene-based flexible electronic devices. Materials Science and Engineering: R: Reports, 2017. 118: p. 1-43.
5. Wei, D., et al., Controllable chemical vapor deposition growth of few layer graphene for electronic devices. Accounts of chemical research, 2013. 46(1): p. 106-115.
6. Sun, Y., M. Sun, and D. Xie, Graphene electronic devices, in Graphene. 2018, Elsevier. p. 103-155.
7. Najim, A., et al., A fundamental study on the electronic and optical properties of graphene oxide under an external electric field. Modern Physics Letters B, 2024. 38(10): p. 2450032.
8. Xie, C., et al., Graphene/semiconductor hybrid heterostructures for optoelectronic device applications. Nano Today, 2018. 19: p. 41-83.
9. Li, X., et al., Graphene in photocatalysis: a review. Small, 2016. 12(48): p. 6640-6696.
10. Xiang, Q., J. Yu, and M. Jaroniec, Graphene-based semiconductor photocatalysts. Chemical Society Reviews, 2012. 41(2): p. 782-796.
11. Xia, B., et al., Recent progress on graphene-based hybrid electrocatalysts. Materials Horizons, 2014. 1(4): p. 379-399.
12. Mazánek, V., et al., Ultrapure graphene is a poor electrocatalyst: definitive proof of the key role of metallic impurities in graphene-based electrocatalysis. ACS nano, 2019. 13(2): p. 1574-1582.
13. Mo, C., R. Yin, and J.R. Raney, Direct ink writing of tough, stretchable silicone composites. Soft Matter, 2022. 18(38): p. 7341-7347.
14. Zhang, K., et al., Molybdenum selenide electrocatalysts for electrochemical hydrogen evolution reaction. ChemElectroChem, 2019. 6(14): p. 3530-3548.
15. Eftekhari, A., Molybdenum diselenide (MoSe2) for energy storage, catalysis, and optoelectronics. Applied Materials Today, 2017. 8: p. 1-17.
16. Acun, A., et al., Germanene: the germanium analogue of graphene. Journal of physics: Condensed matter, 2015. 27(44): p. 443002.
17. Borca, B., et al., Image potential states of germanene. 2D Materials, 2020. 7(3): p. 035021.
18. Ochapski, M.W. and M.P. De Jong, Progress in epitaxial growth of stanene. Open Physics, 2022. 20(1): p. 208-223.
19. Cho, K., J. Yang, and Y. Lu, Phosphorene: An emerging 2D material. Journal of Materials Research, 2017. 32(15): p. 2839-2847.
20. Singh, S., et al. Insights on a new family of 2D material mxene: A review. in AIP conference proceedings. 2021. AIP Publishing.
21. Sun, J., et al., MAX, MXene, or MX: What Are They and Which One Is Better? Advanced Materials, 2023. 35(52): p. 2306072.
22. Li, X., et al., Functional MXene materials: progress of their applications. Chemistry–An Asian Journal, 2018. 13(19): p. 2742-2757.
23. Gogotsi, A.S.a.Y., Raman Spectroscopy Analysis of the Structure and Surface Chemistry of Ti3C2Tx MXene. 2020.
24. Xiong, D., et al., Recent advances in layered Ti3C2Tx MXene for electrochemical energy storage. Small, 2018. 14(17): p. 1703419.
25. Iqbal, A., P. Sambyal, and C.M. Koo, 2D MXenes for electromagnetic shielding: a review. Advanced Functional Materials, 2020. 30(47): p. 2000883.
26. Bhardwaj, R. and A. Hazra, MXene-based gas sensors. Journal of Materials Chemistry C, 2021. 9(44): p. 15735-15754.
27. Lim, J.J.Y. and A.N.K. Lup, Heterostructural TiO 2/Ti 3 C 2 MXene aerogel composite for photocatalytic degradation of palm oil mill effluent. Environmental Science: Advances, 2022. 1(4): p. 570-583.
28. Le, A.T., et al., Mechanisms of removal of heavy metal ions by ZnO particles. Heliyon, 2019. 5(4).
29. Abdullah, F., N.A. Bakar, and M.A. Bakar, Current advancements on the fabrication, modification, and industrial application of zinc oxide as photocatalyst in the removal of organic and inorganic contaminants in aquatic systems. Journal of hazardous materials, 2022. 424: p. 127416.
30. Kamarulzaman, N., M.F. Kasim, and R. Rusdi, Band gap narrowing and widening of ZnO nanostructures and doped materials. Nanoscale research letters, 2015. 10: p. 1-12.
31. Sultana, K.A., et al., Sustainable synthesis of zinc oxide nanoparticles for photocatalytic degradation of organic pollutant and generation of hydroxyl radical. Journal of Molecular Liquids, 2020. 307: p. 112931.
32. Urgessa, Z., et al., Low temperature near band edge recombination dynamics in ZnO nanorods. Journal of Applied Physics, 2014. 116(12).
33. Lin, Y., H. Hu, and Y.H. Hu, Role of ZnO morphology in its reduction and photocatalysis. Applied Surface Science, 2020. 502: p. 144202.

