1Mr. Harshad S. Deshpande, 1Dr. V.B. Jadhav, 2Dr. Mahendra Sahebrao Borse and 3Dr. Ravindra S. Dhivare
1JET’s Z.B. Patil College,Dhule (Maharashtra) -424002.
1JET’s Z.B. Patil College,Dhule (Maharashtra) -424002.
2Department of chemistry, Uttamrao Patil College Dahivel Taluka-Sakri, District-Dhule Maharashtra
3BSSPs Arts, Commerce, and Science college songir Dhule
Email: mahendraborse@yahoo.com and Ravii_1978@rediffmail.com
Abstract: For the preparation of omeprazole suspension granules are available in the market. In this research suspension is prepared by using combination of vehicle, preservative and pH regulator. After preparation of chemical stability is evaluated by using stability indicating parameters. Chemical stability of suspension is significantly increased after pH maintain in strongly basic side. Storage temperature plays significant role in chemical stability. Storage container has no significant impact on chemical stability. Due to use of polysorbet 80, shelf life is increased to significant extent due to its properties such as preservative agent, wetting agent, suspending agent. Research highlights novel suspension medium preparation, its impact on chemical stability. Temperature and storage container impact on stability.
Keywords: Omeprazole suspension, Chemical stability, polysorbet 80, Temperature effect on stability, Noval suspension medium.
Introduction: Proton pump inhibitors commonly known as PPI. PPI is used in the treatment of GRED (Gastroesophageal reflux disease), gastric and duodenal ulcers, erosive esophagitis (1). Omeprazole is wildly used PPI. Omeprazole dosage forms available in market having is lyophilised injection, capsules and dry powder for suspension. Oral route of administration is most common for all medicinal product. However any liquid dosage is always considered as most efficient dosage form, due to having advantages as flexible dose proportionality, suitable for all patient such as elderly or children, high efficiency and quick action. But “Omeprazole” is highly unstable in liquid dosage form due to acid catalyzed and hydrolytic degradation in aqueous medium. Presence of water accelerates protonation resulted to loss in potency. To slow down the reaction speed high pH is maintained by using sodium bicarbonate (2). Refrigerated storage condition further increases stability due to reduction in kinetic energy. In spite of these stabilization process, chemical stability of omeprazole suspension is very less, typically less than 14 days, reflecting the fundamental chemical limitations of maintaining sulfoxide stability in aqueous systems (3).
Hence Noval suspension medium is prepared by using combination of water, polysorbet 80 and NaOH for pH regulation. Polysorbet 80 is surfactant which will be added to increase stability and suspendability due to its wetting, preservation and other properties such as hydrophobic nature, chelating nature, viscosity enhancer etc. but not limited to. (4, 5, 6, 7).
Chemical stability is evaluated mainly as change in appearance, decrease in therapeutic efficacy and increase in impurity. The degradation is influence by light, temperature, reaction with container and closer, oxidation, moisture etc.
Materials and Methods: For the study omeprazole granules are procured from chemist which is manufactured by Dr. Reddy’s laboratories Ltd having brand name Omez Insta. The sachet is having label claim of 20 mg omeprazole. As per instruction provided on the sachet, complete sachet to be dissolved in water. Dubble distilled water is prepared in lab and used. Sodium hydroxide is procured from Merck and Polysorbet 80 (Vicapol 80) is procured from Viaswaat Chemicals.
For performance of test Morter Pestel, glass beaker, measuring cylinder is used to prepare suspension. For storage stability chamber of make Thermolab having storage temperature 2-8°C, 25°C & 60 % RH, 30°C & 75 % RH and 40°C & 75 % RH. For testing,pH meter, Oswal viscometer, Balance and Shimadzu HPLC is used (8).
For the determination of chemical stability of suspension, decided to perform test as Appearance, pH, Viscosity, Specific gravity, Assay, impurities and microbial limit test (8).
After preparation suspension is stored in the glass bottle (Impermeable) and PET bottle (semipermeable) are used (9).
Figer 1 Molecular structure of Omeprazole.
Result Discussion:
Appearance:Evaluatedand foundcolour change indicates progressive degradation, which is more rapid at higher temperatures.
In the cold storage (2-8°C) it starts with off-white, and gradually changes to pale yellow, and eventually yellowish orange. Similar change in colour occurs in all storage temperature. But it will change quickly at elevated temperature such as 30°C & 40°C.
pH: Evaluatedand foundpH decreases with higher temperature and longer storage, consistent with chemical breakdown.At refrigerated condition (2–8°C)pH remains 10.24 compared to initial 11.022, relatively stable.In room temperature (25°C) Slight decline over time to 10.04. At 30°C Noticeable drop 10.1in 45 days.40°C Clear downward trend from 11.02 to 10.31 in just 3 days.
Viscosity: Evaluatedand foundphysical consistency is largely stable, not significantly affected by storage. Across all conditions, viscosity remains in the range 2.4–2.7 mPa·s, showing minor fluctuations.
Density: Evaluatedand founddensity changes are modest, but higher temperatures show more variability. At refrigerated condition (2–8°C) it is ranging from 1.18 to 1.36 g/ml, in room temperature (25°C)slightly higher variation ranging from 1.11–1.42 g/ml. At higher temperature (30°C & 40°C) Variation is less which is 1.18 to 1.39 g/ml, But fluctuation are more.
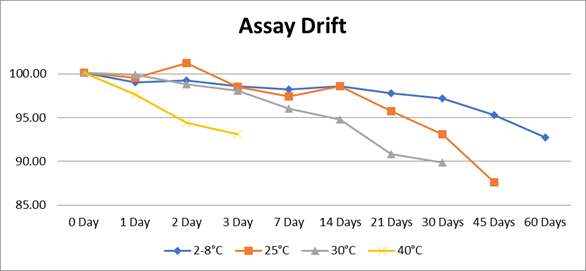
Chart 1: Assay drift at various temperature over the period of storage.
Assay: Evaluatedand found Omeprazole suspension is stable at refrigerated conditions but loses potency faster at elevated temperatures.At refrigerated condition (2–8°C) Gradual decline but remains within 90–100% limit up to 60 days. In room temperature (25°C) assay drops faster and by 30 days it will be about 93 %. In the elevated temperature (30°C) assay falls below 90 % in 30 days. At accelerated temperature degradation reaction will be very fast due to kinetic energy. Very rapid decline observed and at 3 days assay is about 91 %
Impurities: Evaluatedand founddegradation products accumulate significantly at higher temperatures.Impurity D & E are generally below limit (NMT 0.15%), but at higher temperature 30–40°C levels it will approach/exceed thresholds (e.g., Impurity E up to 0.26%). Total impurities remain <0.5% at 2–8°C, but exceed limit at higher temperatures (30°C: 0.53%, 40°C: >0.5%). In the short period of time that is 30 days and 3 days.
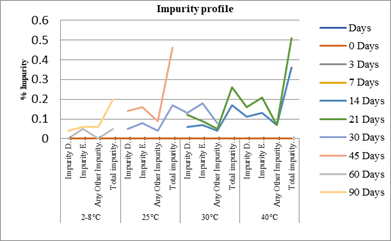
Chart 2: Stability profile of suspension Chart 3 : Impurity profile of suspension
Microbial Limit test: Microbial stability is maintained across all storage conditions. There is no impact of storage microbial susceptibility.
Chart 4: Storage container impact on assay Chart 5: Storage container impact on Impurity
Impact of storage containers are also evaluated and found that, there is no significant change in properties such as appearance, pH, viscosity and specific gravity due to storage container. Hear concluded that Glass bottles consistently show slightly better assay retention across all temperatures. However, there is significant impact on assay find below table for more clarity.
| Temperature | Time Point | Glass Bottle | PET Bottle | Observation |
| 2–8°C | 90 Days | 89.4% | 86.7% | Both within spec; PET slightly lower |
| 25°C | 45 Days | 87.6% | 85.2% | Glass bottle shows better retention |
| 30°C | 30 Days | 89.9% | 88.5% | Solution in glass container is more stable |
| 40°C | 3 Days | 93.1% | 91.7% | Glass maintains potency better |
Table 1: Comparison of impact due to storage containeron assay.
When impurities are compared glass container is found less reactive. It might be due to inert and impermeable nature of glass. However there is no significant difference in the impurity results. The limit of impurities are NMT 0.15 % for impurity D & Impurity E and NMT 0.5 % for Total impurity. Hence concluded that at elevated temperatures (≥25°C), PET bottles show higher impurity accumulation, especially total impurity. Glass bottles perform better in controlling degradation products.
| Temperature | Time Point | Container | Impurity D | Impurity E | Total Impurity | Observation |
| 2–8°C | 90 Days | Glass | 0.04 | 0.06 | 0.2 | All within limits |
| PET | 0.06 | 0.12 | 0.41 | Slightly higher but comparable to glass. | ||
| 25°C | 45 Days | Glass | 0.14 | 0.16 | 0.46 | Near limit |
| PET | 0.15 | 0.19 | 0.56 | Exceeds impurity E and total impurity limit | ||
| 30°C | 30 Days | Glass | 0.13 | 0.18 | 0.46 | Exceeds limit of impurity E |
| PET | 0.19 | 0.16 | 0.53 | All impurities within specification except total impurity. | ||
| 40°C | 3 Days | Glass | 0.16 | 0.21 | 0.49 | Exceeds limit except total impurity. |
| PET | 0.18 | 0.26 | 0.62 | All impurities exceeds limit |
Table 2:Comparison of impact due to storage containeron impurity.
Microbial Load: There is no significant increase in the load during the storage. Can be better understood by following table and graph. After evaluation it is concluded that PET bottles show slightly lower microbial counts across all temperatures.
| Temperature | Time Point | Glass Bottle | PET Bottle | Observation |
| 2–8°C | 90 Days | 34 CFU / Ml | 41 CFU / Ml | Both acceptable |
| 25°C | 45 Days | 22 CFU / Ml | 35 CFU / Ml | PET slightly better |
| 30°C | 30 Days | 19 CFU / Ml | 14 CFU / Ml | PET better |
| 40°C | 3 Days | 21 CFU / Ml | 16 CFU / Ml | PET better |

| Table 2:Comparison of impact due to storage containeron impurity | Chart 6: storage container impact on microbial load |
. Conclusion: Duering studyit is confirmed that the suspension stability is highest in refrigerated (2-8°C) condition, which maintains assay, low impurity and acceptable appearance up to 90 days. The stability found moderate at room temperature which is up to 30 days. At the elevated temperature potency drops below acceptable threshold, and impurities crosses limit threshold in short period and stability is very poor which is only 3 days at 40°C. But the microbial load are well within limit. In the suspension suspendability is maintained throughout the storage period in refrigerated condition (2-8°C) also. When compared containers it is concluded that for cold chain storage (2-8°C) both the containers are suitable. For ambient and elevated temperature glass bottles are preferred due to better impurity control. Though PET bottles offer better microbial resistance but may compromise impurity thresholds and assay retention under stress. The designed solvent for suspension is effective in increasing the physical and chemical stability of suspension.
References:
- WWW.MYOCLINIC.ORG
- Bonfim-Rocha, L., Silva, A. B., de Faria, S. H. B., Vieira, M. F., & de Souza, M. (2020). Production of sodium bicarbonate from CO2 reuse processes: A brief review. International Journal of Chemical Reactor Engineering, 18(1), 20180318.
- Omari, D. M., Akkam, Y., & Sallam, A. (2021). Drug-excipient interactions: an overview on mechanisms and effects on drug stability and bioavailability. Annals of the Romanian Society for Cell Biology, 25(4), 8402-8429.
- Aulton, M. and Taylor, K. (2013). Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, (4th ed.). Edinburgh: Churchill Livingstone.
- Chaudhari, S. and Patil, P. (2012). Pharmaceutical Excipients: A review. International Journal of Advances in Pharmacy, Biology and Chemistry, 1(1): 21-34.
- Kulshreshtha A., Singh O. and Wall M. (2010). Pharmaceutical Suspensions: From Formulation Development to Manufacturing.London, New York, Dordrecht Heidelberg: Springer.
- Attwood, D., Florence, A.T. (1983). Surfactants in suspension systems. In: Surfactant Systems. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-5775-6_9.
- Stability testing of new drug substances and drug products (ICH Q1 A (R2)).
- Sandra B.M. Jaime, Rosa M. V. Ales, Paula F. J. Bocoli (2022) Moisture and oxygen barrier properties of Glass, PET and HDPE bottles for pharmaceutical products. Journal of drug delivery science and technology 71 (2022) 103330.
- European Pharmacopeia monograph (Ph. Eur. monograph 1032)

