Hitesh N. Wankhedea, Harshal S. Gawaleb, Rajendra R. Ahirea, Anup J. Morea,*
aDepartment of Physics, VVM’s S.G.PatilArts,Science and Commerce College Sakri 424304 Dist. Dhule, KBC NMU Jalgaon, Maharashtra, India
bDepartment of Physics, JET’s Z.B.Patil college, Dhule 424002, KBC NMU Jalgaon, Maharashtra, India
Abstract
The growing global demand for energy has intensified the need for advanced and efficient energy storage technologies. Supercapacitors and batteries have gained considerable interest due to their essential role in modern energy storage systems. The effectiveness of these devices largely depends on the characteristics of the electrode materials, such as high specific capacitance, superior electrical conductivity, large surface area, abundant availability, and favorable electrochemical properties. While cobalt-based nanomaterials offer high conductivity, abundant resources, and strong capacitance performance for supercapacitor electrodes, limitations such as structural degradation and insufficient power density remain unresolved. This paper reviews on advances in cobalt Sulfide based nanomaterials electrode materials for supercapacitors, with a focus on their preparation methods, electrochemical performance and properties. It focuses on methods to enhance the electrochemical performance of these materials. It shows that synergistic effect can improve the morphology of nanomaterials can significantly boost their performance, with mesoporous structures. Key findings from the literature on batteries and supercapacitors are summarized, highlighting Cobalt sulfide-based materials integrated with carbon nanotubes, graphene, reduced graphene oxide, MAX phase (Class of 2D inorganic compounds comprising atomically thin layers of transition metal carbides, nitrides, or carbonitrides) shortly known as MXene, Metal Organic Framework(MOF), nickel foam and metal elements such as nickel, manganese, etc.
Keywords:Supercapacitor, cobalt composites, specific capacitance, energy density,nanomaterials, hydrothermal
1. Introduction
After the industrial revolution demand of energy completely rely on energy extracted from fossil fuel (oil, gas and coal) but it causes a severe effect on human health like cardiovascular disease, respiratory syndrome, cancer, reproductive effects, etc. and it can happen due to the evolution of carbon dioxide, carbon monoxide, CFC, and other toxic gases which may leads to greenhouse effect. To get ride from this problem we need to adopt renewable energy resources like hydroelectric energy, solar energy, wind energy, geothermal energy, tidal energy, and biomass energy.[1] There is a challenge in effectively storing energy extracted from these resources. To address this issue electrochemical energy storage system (EES), namely supercapacitors and batteries have become crucial technologies.[2] Energy density of batteries is higher than the supercapacitor but power density of batteries is lower than the supercapacitor so for rapid charging and discharging applications supercapacitor are more convenient. Continuous research progression in this area is due to wide range of applications such as industries, medical field, military, automobile sector, etc.
In recent days automobile sector mostly relies on lithium-ion batteries due to higher energy density and safe during handling. Lithium is a key part of batteries that runs electric vehicles but due to limited availability of lithium it really hard to keep up with demand and supply. Researchers are continuously working on replacement of lithium to alkali metals like sodium cause abundant in nature and low cost but sodium ion batteries having poor cyclic performance.[3] In comparison to batteries supercapacitor having some positive features like fast charging- discharging cycles. Supercapacitor require 1-10 s and batteries require 0.5-5 hr. charging -discharging time. Power density defines how quickly energy can be delivered or receive per unit mass (W/kg): supercapacitor having higher power density 500-10000 W/kg and batteries having power density less than 1000 W/kg. Supercapacitor have longer lifetime more than 500,000 hrs. and batteries 500-1000 hrs. Energy density defines amount of energy stored per unit mass: energy of batteries 10-100 Wh/kg more than supercapacitors 1-10 Wh/kg.[4] The Ragone plot shown in graph 1. provide the information about behavior of electrochemical energy storage devices power density and energy density.[5]
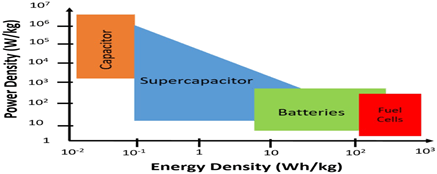
Graph 1. Ragone plot of different electrochemical energy conversion systems.[5]
Conventional capacitor having lowest energy density and higher power density in comparison to other electrochemical energy storage devices. Supercapacitor having lower energy density and higher power density also batteries having higher energy density and lower power density compared to other electrochemical devices.[6] To overcome the limitations of conventional batteries, supercapacitors have emerged as a promising electrochemical energy storage device. Unlike batteries, supercapacitors require electrode materials that exhibit high electrical conductivity, a large electrochemically active surface area, and well-tuned porosity to facilitate rapid ion transport. In addition, excellent thermal and chemical stability of the electrode material is essential to ensure long-term performance and safety. The development and fabrication of such advanced electrode materials play a crucial role in enhancing the energy density and overall efficiency of supercapacitor systems.
2. Synthesis Method
Cobalt sulfide (CoS) can be synthesized through several methods, depending on the desired properties and the form of the material. In this review article most of the materials are synthesize by hydrothermal method, solvothermal method, microwave induced synthesis, chemical bath deposition (CBD) etc.
2.1 Hydrothermal Method
This is a popular method for synthesizing CoS nanostructures, such as nanoparticles, nanowires, nanotubes etc. It involves a chemical reaction in an aqueous solution at elevated temperature and pressure. It involves crystalizing materials from aqueous solutions at high temperatures and pressures within a sealed and compact vessel. This method has some advantages facilitates the growth of nanostructured materials with controlled morphologies.
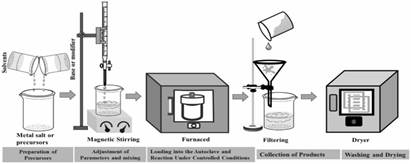
Fig. 1. Schematic representation of hydrothermal synthesis method[7]
It provides precise control over particle size and morphology, form crystalline structures at relatively low temperatures and allows to enhance material properties. This method also has limitations, requires specialized equipment to withstand high pressures. Extended reaction times may be necessary to achieve desired crystallinity.[7]
2.2 Solvothermal Method
In this method chemical reaction carried out in an autoclave which sealed vessel using a different solvent at high temperature and controlled pressure. Due to this conditions nucleation and growth of materials of materials occurred in a controlled manner. This method having some advantages, it allows precise control over particle size, crystallinity, shape and growth of pure materials. It suitable for synthesis of wide range of nanostructure of metal sulfides, oxide and hydroxide.[8]This method has some limitations like the process is time consuming and it run with help of high pressure; scalability is also a problem.
2.3 Microwave-Assisted Synthesis Method
Microwave-assisted synthesis involves rapid heating of reactants using microwave radiation, enabling uniform nucleation and growth of nanomaterials. Heating occurs due to the interaction of 2.54 GHz microwave energy with polar molecules and ions via dipole polarization and ionic conduction mechanisms. This technique offers reduced reaction time, uniform heating, and high energy efficiency. However, limitations include restricted precursor selection and challenges in large-scale production.[9]The method has been effectively applied to synthesize metal sulfide nanoparticles, metal oxide nanoparticles etc. for high-performance supercapacitor electrodes.
2.4 Chemical Bath deposition (CBD) Method
The chemical bath deposition (CBD) technique is a low-cost and simple method used to deposit thin films of materials from a solution. In this method, the substrate is immersed in a chemical bath containing metal ions and a suitable complexing agent. Controlled chemical reactions in the solution lead to the slow and uniform deposition of the material onto the substrate surface. The deposition occurs due to the controlled release of ions and subsequent nucleation on the substrate. Parameters such as bath temperature, pH, concentration of reactants, and deposition time play an important role in determining the thickness, morphology, and quality of the deposited film. CBD has several advantages. It is simple, cost-effective, and does not require vacuum or high-temperature conditions. It allows large-area and uniform film deposition and is suitable for coating complex-shaped substrates.[10] However, the technique has some limitations, such as poor adhesion, lower crystallinity, and limited control over film thickness compared to advanced deposition methods.
3. Electrochemical performance analysis
In recent years metal sulfide and oxides-based electrodes materials are more prominent for supercapacitor applications due to their excellent redox reversibility, high electrical conductivity, excellent morphology, and high specific capacitance. This review article focuses on cobalt sulfide-based electrode materials for supercapacitor application.[11,12] Different techniques are used to tune the morphology of various materials including hydrothermal, solvothermal, supercritical fluid synthesis, CBD, microwave assisted synthesis technique. Morphology of materials can be responsible for effective energy storage and improve the electrochemical performance of electrode materials. In this article a few cobalt sulfide-based electrode materials, some remarkable morphologies like nanowires, nano-tubes, nano-sheets,flakes, and nano-flower-like structures have been reported. A silver fungus-like cobalt sulfide (CoS) nanostructure was successfully synthesized via solvothermal method and use as an electrode material for high-performance supercapacitors. The unique fungus-like morphology provides a large active surface area and abundant electroactive sites, which enhance electrolyte penetration and facilitate fast charge transport. As a result, the silver fungus-like CoS (SFC) electrode exhibits a high specific capacitance of 350.4 F g⁻¹ at a current density of 1 A g⁻¹. The device SFC//AC delivers an energy density of 45.2 Wh kg⁻¹ at a power density of 1500 W kg⁻¹, provides excellent energy storage capability.[13] A cobalt sulfide nanoparticles synthesize by hydrothermal route and calcinated at 200 0C for 1 hr. form a hexagonal phase of CoS. As a result, the CoS electrode delivers a high specific capacitance of 285.8 F g⁻¹ at a current density of 2 A g⁻¹. Furthermore, the electrode demonstrates excellent cycling stability, retaining 96% of its initial capacitance even after 5000 galvanostatic charge–discharge cycles, indicating strong structural integrity and reversibility. The device made up of CoS/CC//AC achieves an energy density of 25.8 Wh kg⁻¹ andhigh-power density of 14,800 W kg⁻¹, highlighting its capability to store substantial energy while delivering it rapidly.[14]Nickel cobalt sulfide is high promising material electrode for supercapacitor application good cycling stability. NCS-180 synthesize at 180oC display urchin like crystalline structure provide more electroactive sites and good electrochemical performance.Owing to these structural advantages, the NCS-180 electrode delivers a high specific charge capacity of 664.30 C g⁻¹ at a current density of 1 A g⁻¹, indicating better Faradaic charge-storage capability. The electrode demonstrates good long-term cycling stability, retaining 93.30% of its initial capacity after 6000 galvanostatic charge–discharge cycles, provides structural stability during repeated cycles. NCS-180//AC system achieves an energy density of 50.35 Wh kg⁻¹ with a corresponding power density of 750 W kg⁻¹.[15]Dumb-bell shaped 10-20 nm sized cobalt sulfide (CoS) particle prepared by solvothermal route exhibit specific capacitance of 310 F/g at current density of 5 A/g and 95% of capacitance retention after 5000 charge–discharge cycles. Device made up of Cos//AC provide specific capacitance of 5.3 Wh kg⁻¹ and a high-power density of 1800 W kg⁻¹ with an excellent electrochemical stability.[16] High-performance nickel–cobalt sulfide–terephthalic acid (NCS–BDC) composite electrode synthesized via a simple solvothermal route for energy storage devices like supercapacitor. Highly mesoporous structure creates more active site for reaction and provides more surface area for transmission of ions. NCS-BDS has good electrochemical properties. It has specific capacitance 1267.25 F g⁻¹ at a low current density of 0.5 A g⁻¹. Electrode shows good cycling stability, maintain 92% of its initial capacitance after 5000 charge–discharge cycles. NCS-BDC based device achieved high energy density 52.29 Wh kg⁻¹.[17] Hydrothermal route utilizes to synthesize cobalt sulfide/reduced graphene oxide (Co3S4/rGO) nanocomposite. As a result, the CoS/rGO nanocomposite provide an ultrahigh specific capacitance of 1560 F g⁻¹ at a current density of 1 A g⁻¹ and also the electrode exhibits good cycling stability, retaining 89% of its initial capacitance after 5000 charge–discharge cycles. Device achieves an energy density of 40.2 Wh kg⁻¹ and a power density of 804 W kg⁻¹.[18]
CoS nanosheet fabricated on metal organic framework on nickel foam (NF) by hydrothermal route. CoS/NF electrode display a high specific capacity 1359 C g−1 at the current density of 2 A g−1, and excellent cycling stability of 89.4% after 4000 cycles. A device fabricated by CoS/NF positive electrode and AC as a negative electrode shows high energy density of 57.4 W h kg−1 at a power density of 405.2 W kg−1.[19]CoS/MXene was synthesize by supercritical fluid synthesis method. Electrochemical performance of CoS/MXene,CoS/MXene/PANI and CoS/MXene/PEDOT was studied. CoS/MXene/PANI electrode delivered specific capacitance of 407 F g⁻¹ at current density of 2 A/g with cycling stability of 97% after 10000 cycles. Also, CoS/MXene/PANI electrode delivered specific capacitance of 630 F g⁻¹ at current density of 2 A/g with cycling stability of 96% after 10000 cycles useful for supercapacitor application.[20]Ni-based flower-like nitrogen-rich carbon (NCNi) synthesized on a carbon felt (CF) substrate through a hydrothermal route. EC-NiCoS@NCNi@CF electrode shows specific capacitance of 190.78 F g⁻¹ at current density of 0.5 A/g having cycling stability of 92.2% after 4000 cycles. Device delivered energy density of 64.77 W h kg−1 and power density of 420.13 Wkg−1.[21]Co-Ni-S composite electrode prepared through a two-step process involving electrodeposition followed by hydrothermal sulfurization which brings more cobalt active sites for redox reaction.The Co-Ni-S composite electrode delivers high specific capacitance of 3586 F g−1 at 1 A g−1 and 97% capacity retention over 5000 cycles.[22]The rGO/NCS/PANI electrode provide a high specific capacitance of 628 F g−1at a current density of 10 A g−1 and retentivity of 84 % after 5000 charge-discharge cycles showing excellent cycling stability.[23] Two-stage hydrothermal method used to synthesize nickel–cobalt sulfide nanostructures to enhance the electrochemical properties of materials. Electrode achieve specific capacitance of 8.1 F cm-2 at current density 5mA cm-2. Nickel–cobalt sulfide electrodes as the positive electrode and activated carbon as the negative electrode delivered high energy density of 51.2 Wh kg−1 at a power density of 262.5 W kg−1.[24] By utilizing different reaction conditions Nickel cobalt sulfide (NCS) microspheres are successfully synthesized by an easy one-step hydrothermal method . NCSW-200 electrode delivered a specific capacitance of 369 F g−1 at current density of 0.5 A g−1 having capacitive retention of 67% after 2000 cycles.[25] Two step facial hydrothermal method used to synthesize nickel cobalt sulfide nanoparticles (NCS) deposited on nitrogen and sulfur doped graphene which provides a synergistic effect and improve electrochemical parameters. Electrode delivered a specific capacitance of 630.6 F g−1 at 1 A g−1 current density with retention of 110 % after 10000 cycles. Also, energy density of 19.35 Wh kg−1 at a power density of 235.0 W kg−1 showing exceptional capacity for supercapacitor application.[26]
Ni-Co-S/Co(OH)2 electrode synthesize by two step facial method with synergistic effect provides excellent electrochemical performance shows a specific capacitance of 1560.8 F g−1 at 1 A g−1 current density with retention of 88% after 10000 cycles. A device shows high energy density of 48.8 W h kg−1 at a power density of 800 W kg−1 with excellent cycle stability.[27] Hydrothermal route employed for successfully synthesis of NiCo₂S₄ polyhedral structures for application to supercapacitor and lithium-ion battery. Electrode exhibit a specific capacitance of 1298 F g−1 at 1 A g−1. Capacity retention of 90.44% after 8000 cycles.[28] Etching/ ion exchange method used to synthesize Ni-Co-S nanosheets on activated carbon cloth for fabrication of supercapacitor application.The Ni-Co-S/ACC electrode can deliver a specific capacitance of 2392 F g−1 at the current density of 1 A g−1 and also have retentivity of 82 % after 10000 cycles. Device Ni-Co-S/ACC as positive electrode and activated carbon as negative electrode display high energy density of 30.1 Wh kg−1 at power density of 800.2 W kg−1.[29] Hierarchical NiCo2S4@Co(OH)2 nanotube structure on Nickel foam have been synthesized through a facial method. Synergistic effect of NiCo2S4 nanotubes and Co(OH)2 nanosheets delivered a superior electrochemical performance having specific capacity of 9.6 F cm-2 at current density of 2 mA cm-2 with capacitive retention of 70.01% after 5000 cycles.[30]One-step hydrothermal method utilize for the synthesis of the flaky attached hollow-sphere structure NiCo2S4 electrode materials.The NCS-10 electrode atPh 10 shows an excellent specific capacitance of 1366 F g−1 at the current density of 1 A g−1 at high retention of 89.8% after 2000 cycles.[31]The poor performance and cyclic stability of the materials have limit practical applications so need to improved quality of electrode by improving morphology. Carbon flakes with an ultrahigh surface area prepared from eggplant utilize as a substrates to enhance the electrical conductivity of NiCo2S4 nanosheets. Exhibit a specific capacitance of 1394.5 F g−1 at 1 A g−1 and cyclic stability of 124% after 10000 cycles. Delivered a high energy density of 46.5 Wh kg−1 at a power density of 801 W kg−1.[32]For high performance supercapacitor require high specific surface areas, high redox active sites, efficient electrons-ions migration channels. Facial two step hydrothermal route used to fabricate highly porous Co3S4@Ni3S4 heterostructure nanowire arrays prepared onto Ni foam.Delivered specific capacitance of 3.6 F cm-2 at energy density of 0.8 mA cm-2get 80% capacitive retention after 5000 charge-discharge cycles.[33]Hydrothermal method and potentiostatic deposition utilize to grow hierarchical polyaniline-coated NiCo2S4 nanowires on carbon fiber “NiCo2S4@PANI/CF”. NiCo2S4@PANI/CF material electrode have multiple electroactive sites so it enhances electrochemical performance of electrode as well as device. Electrode display high specific capacitance value 1823 F g−1 at 2 mA cm-2 and excellent cycling stability of 86.2% after 5000 cycles. Device NiCo2S4@PANI/CF delivers a high energy density of 64.92 Wh kg−1 at a power density of 276.23 W kg−1.[34]NiCo2S4, a spinel-structured has a high specific capacity, it has promising characteristic of electrode material for supercapacitors but due to poor electrical conductivity need to tune its morphology. In this work NiCo2S4 deposited on the surface of carbon nanotubes (CNTs) to enhance the electrical conductivity. CNTs@NiCo2S4 delivered specific capacitance of 216.4 mAh g−1 at 1 A g−1with cyclic retention of 75 % after 2000 cycles.[35]Microwave assisted technique is used to synthesize NCS/CNTs-H electrode followed by post annealing to anchor NCS nanoparticles on multiwall CNTs. This structure enhances electrochemical performance of electrode; it delivered high specific capacitance of 1261 F g-1 at 1 A g-1 with retention capability of 84.4%. Device NCS/CNTs-H//AC deliver a high energy density 58.4 Wh kg-1 at the power density of 400 W kg-1. NCS/CNTs-H offer good electrochemical performance so it stands high for supercapacitor electrode.[36]Microwave assisted technique utilizes to synthesis of honeycomb-like NCS/graphene composites which use as ultrahigh supercapacitor electrode. NCS/G-H exhibit high specific capacitance of 1186 F g-1 at 1 A g-1 and cyclic retention of 89.8% and delivered energy density of 46.4 Wh kg-1.[37]Sonochemical method used for synthesis of cobalt sulfide nanomaterial and cobalt phosphate nanoflakes and composite of both form a CoS/Co3(Po4)2 electrode. Composite consisting 75% of CoS and 25% of Co3(Po4)2 composition, shows a specific capacitance of 728.2 F g-1 at current density of 0.6 Ag-1 with capacitive retention of 95.10% after 5000 cycles. Device provides remarkable specific energy of 63.93 Wh kg-1 along with specific power of 850 W kg-1at 1 Ag-1.[38]MnCo2S4@CoNi LDH core shell heterostructure synthesis on nickel foam using hydrothermal reaction and electrodeposition technique. MnCo2S4 nanotubes provide excellent electrical conductivity whereas CoNi LDH nanosheets provide more electrochemical active sites for better supercapacitive performance. The electrode provides a specific capacitance of 1206 C g−1 at 1 A g-1 and excellent cycling performance with 92% retention after 10 000 cycles.[39]Cobalt sulfide nanostructure synthesizes by one step hydrothermal method for different temperature ranging from 160oC to 220oC. Sample get high crystallinity and hexagonal structure at 220oC. A high specific capacitance deliver of 1583 F g-1 at a current density of 1 A g-1 with good cyclic performance for supercapacitor application.[40]
Sheet-like nickel cobalt sulfide nanoparticles synthesize by a two-step hydrothermaltechnique provide rich sulfur vacancies.NiCo2S4 nanosheets provide good specific capacitance of 971 Fg-1 at 2 A g-1 and an excellent cyclic stability of 88.7% after 3500 cycles.[41] By facial solvothermal method mixed nickel-cobalt sulfide (NCSs) prepared for supercapacitor application. The mixed NCS prepared at a nickel: cobalt molar ratio of 3:1 exhibited a specificcapacitance of1345 Fg-1 at a current density of 2 A g-1 with 95% of its initial capacitance after 3000 charge-discharge cycles.[42] Cobalt sulfide composes with different metals such as copper Cu and manganese Mn fabricated by hydrothermal method on nickel foam provide a unique morphology of nanoflakes of different texture. Mn-CoS-3/NF boost the specific capacitance of 2379 F g-1 at 1 A g-1 with capacitance retention about 65% after 5500 cycles comparing to 48% of CoS-3/NF and 55% Cu-CoS-3/NF. Mn-CoS-3/NF deliver high surface area, low internal resistance, flaky nanostructure. Mn-CoS-3/NF//AC/NF device deliver energy density of 17.94 Wh kg-1 and power density of 6405 W kg-1.[43] Twostep hydrothermal method used to synthesis of cobalt sulfide layered flower-like morphology binder-free Co9S8 electrodes deposited onto nickel foam with an enhanced specific capacity of 1611.87 F g-1at 1 mA cm–2.[44]CoS/G nanocomposite successfully synthesize by one pot hydrothermal method. CoS nanosphere offers specific capacitance of 390 F g-1 and CoS on graphene shows excellent specific capacitance 739.83 F g-1 with capacitance retention of 91.2 % after 3000 cycles.[45] Dandelion likeNiCo2S4@PPy/NF microsphere synthesize by hydrothermal method. Electrode shows remarkable specific capacitance of 2554.9 F g−1 at 2.54 A g−1 with capacitive retention of 92% after 10000 cycles. Device delivered an energy density of 35.17 Wh kg−1 at a power density of 1472 W kg−1.[46]Electrochemical performance of MXene tune by CoS synthesize on Mxene by one step solvent thermal method. Delivered a specific capacitance of 1320 F g−1 at a current density of 1 A g−1 and it shows cyclic performance with 78.4% after 3000 cycles device delivered an energy density 28.8 Wh kg-1 and 800 W kg-1.[47] A simple two step hydrothermal process utilize to prepared a binder-free graphene-nanosheets wrapped Co3S4 hybrid electrode is prepared on conductive Ni-foam. structure of the Co3S4-rGO shows a specific capacitance of 2314 F g−1 with 92.6% cyclic stability after 1000 cycles. Device delivered energy density of 54.32 Wh kg-1and power density of 6250 W kg-1.[48]A two-step hydrothermal method uses to synthesize nickel and cobalt sulfide with different ratios of nickel and cobalt. NC24 sample with the Ni/Co ratio of 1:2 hollow nanotube arrays composed of NiCo2S4 provides nanorod array structure which gives excellent specific capacitance of 1527 C g−1 at 1 A g−1 with capacitive retention of 93.81% after 2000 cycles. Symmetrical supercapacitor from this electrode delivers high energy density of 67.5 Wh kg-1.[49]
A simple chemical bath synthesis methodutilizesto synthesize flaky nickel cobalt sulfides (NiCoxSy) materials display specific capacitance of 1196.1 F g−1 at 1 A g−1 with cyclic retention of 97.5% after 4000 cycles.[50] A novel urchin-like hollow nickel cobalt sulfide (NiCo2S4) fabricated by a facile template-free methodthis structure improves electrochemical performance of electrode as well as device. Electrode display a specific capacitance of 1398F g−1 at 1 A g−1 with excellent cyclic stability of 74.1% after 5000 cycles.[51]Flower like NiCo2S4 prepared by rapid chemical precipitation assisted annealing method deliver a specific capacitance of 2198.9 F g−1 at 1 A g−1. A device NiCo2S4//AC deliver a high energy density of 38.2 Wh kg−1 at power density of 400 W kg−1.[52] One step hydrothermal method used to fabricate reduced graphene oxide/nickel-cobalt sulfide (rGO/NiCo2S4). Needle like structure of NiCo2S4 have many nanoparticles very well adhered to reduce graphene oxide. Prepared electrode has porosity and it leads to excellent conductivity possess a specific capacitance of capacitance of 813 F g−1 at 1.5 A g−1 with good cyclic stability of 84.3% after 2000 cycles. Device shows a high energy density of 40.3 Wh kg−1 and power density of 375 W kg−1.[53] Hydrothermal method used to fabricate NiCo2S4 nanorodon nickel foam (NF). It shows excellent specific capacitance of 3093 F g−1 at 5 A g−1 with cyclic stability of 41.7% after 2000 cycles. Device shows a high energy density of 39.3 Wh kg−1 and power density of 800 W kg−1.[54] A facial two step chemical bath deposition technique used to synthesize a NiCo2S4 nanowire arrays grown on 3D graphene foams (3DGF) for supercapacitor application. It offers a high specific capacitance of 1454.6 F g−1 at 1.3 A g−1 with cycling stability of 96% after 3000 cycles.[55] Hydrothermally synthesize Cobalt sulfide Co3S4 nanosheet decorated with nitrogen doped carbon dots featuring rich sulfur vacancies and copper doping (V-Cu-Co3S4/NCDs). It delivered a specific capacitance of 619.2 C g−1 at 1 A g−1 with capacitive retention of 86.9% after 10000 cycles.[56] An electrodeposition hydrothermal techniqueuses to deposit NiCo2S4 nanoarrays on carbon nanofibers with different morphologies, carbon nanofibers have high surface-area-to-volume ratios, excellent mechanical strengths, and remarkable flexibilities so it provides anexcellent electrochemical property. NCS@C shows a specific capacitance of 334.7 mAh g−1 at current density of 2 A g−1 and the device exhibited high energy and power densities of 12.91 Wh kg−1 and 358 W kg−1.[57] Hydrothermal synthesis of cobalt sulfide nanoparticle on carbon cloth with varying precursor ratios, hydrothermal temperature and time. Structural analysis confirms the formation of hexagonal phase of CoS.Co:S ratio of 1:2 at 1600C for 15 h exhibited the highest specific capacitance of 424 F g-1 at 1 A g-1 with excellent cyclic stability of 90% after 1000 cycles.[58] Hydrothermal method uses to prepared NiCo2S4 flower-shaped crystal nickel–cobalt sulfide on nickel foam. It shows a specific capacitance of 3867.8 F g-1 at 1 A g-1 with cyclic retention of 90.57% after 2000 cycles.[59]
Table.1. Highlight electrochemical parameters of Cobalt sulfide-based electrodes.
Hydrothermal treatment utilizes to fabricate cobalt sulfide (Co3S4) from cobalt oxide as a precursor for 20 hr. duration and it’s a more suitable for super capacitor application as a cathode. It exhibits a specific capacitance of 480.40 F g-1 at 1 A g-1.[60]
Cobalt sulfide–based materials and their composites exhibit high specific capacitance values, making them promising candidates for super capacitor electrode applications. Graph 2. below illustrates the energy density achieved by various cobalt sulfide–based electrodes, the energy density indicates how much energy a super capacitor can stored per unit mass. Energy density of these materials can be effectively tuned by selecting suitable substrates and combining cobalt sulfide with other functional materials. Graph 3. Below illustrates the power density of cobalt sulfide-based electrodes, power density indicates how quickly stored energy can be delivered.
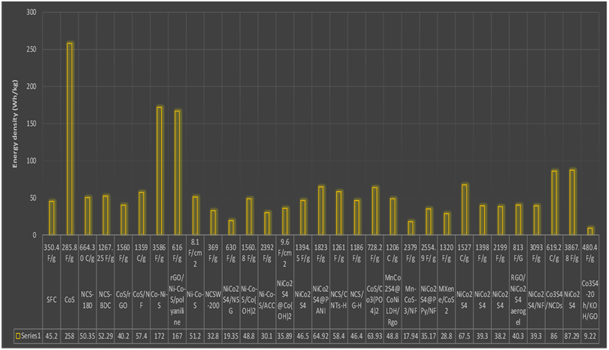
Graph 2. Represent energy density of Cobalt based electrodes.
Graph 3. Represent power density of Cobalt based electrodes.
4. Conclusion
Cobalt sulfide- based nanomaterials are promising supercapacitor electrodes owing to their high redox activity, good conductivity, tunable nanostructures. Morphology control and synergistic engineering through composites and heterostructures significantly enhance electrochemical performance, while future efforts should focus on scalable synthesis and long-term device stability.
5. References
- J. A. Goudar, S. N. Thrinethra, S. Chapi, M. V. Murugendrappa, M. R. Saeb, M. S. Kalajahi, Adv. Energy Sustainability Res., 2025, 6, 2400271.
- F. Ali, X. Liu, D. Zhou, X. Yang, J. Xu, T. Schenk, J. Müller, U. Schroeder, F. Cao, X. Dong, J. Appl. Phys.,2017, 122, 144105-1-7.
- L. Yang, Q. Zhu, K. Yang, X. Xu, J. Huang, H. Chen, H. Wang, Nanomaterials, 2022, 12, 4065.
- W. Raza, F. Ali, N. Raza, Y. Luo, K. H. Kim, J. Yang, S. Kumar, A. Mehmood, E. E. Kwon, Nano Energy, 2018, 52, 441-473.
- I. S. Ismail, F. H. O. Muhamad, N. A. Rashidi, S. Yusup, Biomass Conversion and Biorefinery, 2023, 13, 14341-14357.
- G. Wang, Z. Lu, Y. Li, L. Li, H. Ji, A. Feteira, D. Zhou, D. Wang, S. Zhang, I. M. Reaney, Chem Rev., 2021,121, 6124–6172.
- T. Q. Tazim, Md. Kawsar, Md. S. Hossain, N. M. Bahadur, S. Ahmed, Next Nanotechnology, 2025, 7, 100167.
- Y. Huo, S. Xiu, L.Y. Meng , B. Quan, Chemical Engineering Journal, 2023, 451, 138572.
- S. Li, H. Ma, P.Ouyang, Y. Li, Y. Duan, Y. Zhou, W. Ong, F. Dong,Green Energy &Environment, 2025, 10, 1597-1623.
- V.S. Nalawade, R.S. Redekar, A.A. Bhoite, K.V. Patil, N.L. Tarwal, V.S. Kumbhar, S.M. Pawar,Inorganic Chemistry Communications, 2024, 170, 113281.
- K. Subramani, D. Jeyakumar and M. Sathish, Phys. Chem.Chem. Phys., 2014, 16, 4952–4961.
- K. Subramani, N. Lakshminarasimhan, P. Kamaraj, M. Sathish, RSC Adv., 2016, 6, 15941–15951.
- H. Wang, Journal of Energy Storage, 2021, 40, 102764.
- L. A Miya, S. K. Ghosh, P. Kumari, C. N. M. Morema, K. Mallick, Chemical Papers, 2025, 79, 7617–7631.
- Y. Lei, Q. Xu, Y. Miao, J. Shi, Mater. Adv., 2025, 6, 7427–743.
- K. Subramani, N. Sudhan, R. Divya, M. Sathish, RSC Adv., 2017, 7, 6648–6659.
- D. Loganathan, D. P. Nambia, ACS Appl. Electron. Mater, 2026, 8, 558–569.
- U. Bharathy R, G. Rajamanicham, M. Deshpande, Jothika B, International Journal of Hydrogen Energy, 2025,129, 38-50.
- F. Nasiri, L. Fotouhi, S. Shahrokhian, M. Zirak, Scientific Reports, 2024,14, 6045.
- Chetana S, S. Upadhyay, N.C.Joshi, N.Kumar, P. Choudhary, N. Sharma, V.N.Thakur,Chemistry of Flat Chemistry, 2023, 37, 100456.
- R. D. Abdoljabbar, H. Sharifpour, A. Kulkarni, S. Shahrokhian, D. P. Dubal, ACS Appl. Energy Mater. 2025, 8, 2779–2794.
- S. Prabu, S. Mohanapriya, B.K. Chang,M. R. Pallavolu, K.Y. Chiang, J. Mater. Chem. A, 2025,13, 38065-38079.
- A. Barhoi, A. Chowdhury, C. Bora, S. Hussain, Journal of Energy Storage, 2025, 133, 118033.
- A. A. Markhabayeva, A. S. Anarova, K. A. Abdullin,Z. K. Kalkozova, A. T. Tulegenova, N. Nuraje, Phys. Status Solid RRL, 2023,18, 2300211-1-9.
- M. Shah, P. Kour, S. Kour, A.L. Sharma, Materials Chemistry and Physics, 2024, 325, 129797.
- N. Poompiew, P. Pattananuwat, P. Potiyaraj, RSC Adv., 2021, 11, 25057–25067.
- T. Xu, G. Li, L Zhao, Chemical Engineering Journal, 2018, 336, 602-611.
- G. Xiang, Y. Meng, G. Qu, J. Yin, B. Teng, Q. Wei, X. Xu,Science Bulletin, 2020, 65, 443-451.
- W. Zhao , Y. Zheng , L. Cui, D. Jia, D. Wei , R. Zheng, C. Barrow ,W. Yang , J. Liu , Chemical Engineering Journal, 2019, 371, 461-469.
- R. Li, S. Wang, Z. Huang, F. Lu, T. He, Journal of Power Sources, 2016, 312, 156-164.
- C. Wei, Y. Huang , S. Xue, X. Zhang, X. Chen , J. Yan , W. Yao, Chemical Engineering Journal, 2017, 317, 873-881.
- Y. Liu, Z. Li , L. Yao , S. Chen, P. Zhang , L. Deng,Chemical Engineering Journal, 2019, 366, 550-559.
- Z. Gao , C. Chen , J. Chang, L. Chen, P. Wang, D.Wu, F. Xu, K. Jiang , Chemical Engineering Journal, 2018, 343, 572-582.
- X. Liu, Z. Wu, Y. Yin, Chemical Engineering Journal, 2017, 323, 330-3339.
- R. Bian, D. Song, W. Si, T. Zhang, Y. Zhang, P. Lu, F. Hou, Dr. J. Liang, ChemElectroChem, 2020, 7, 3663-3669.
- J. Zou, D. Xie, F. Zhao, H. Wu, Y. Niu, Z. Li, Q. Zou, F. Deng, Q. Zhang, X. Zeng, Journal of Materials Science, 2021, 56, 1561-1576.
- F. Zhao, D. Xie, W. Huang, X. Song, A.Z.G.Muhammad , H. Wu, F. Deng, Q. Zhang, J. Zou, X. Zeng ,Journal of Colloid and Interface Science, 2020, 580, 160-170.
- Z. I. Muhammad, J. Khan, A. Muhammad Afzal , S. Aftab , Electrochimica Acta, 2021, 384, 138358.
- H. Liang,T. Lin, S. Wang,H. Jia,C. Li, J. Cao,J. Feng,W. Fei, J. Qi, Dalton Trans., 2020,49, 196-202.
- A. Alshoaibi, Materials, 2023, 16, 4512.
- Y. A. Kumar, A. A. Yadav, B. Ali Al-Asbahi, S.W. Kang, Md. Moniruzzaman, Molecules 2022, 27, 7458.
- T.V.Nguyen, L.T. Son, P.M. Thao, L.T. Son, D.T. Phat, N.T. Lan, N.V. Nghia , T.V.Thu, Journal of Alloys and Compounds, 2020, 831, 154921.
- G. Surender, F.S. Omar, S. Bashir, M. Pershaanaa, S. Ramesh, K. Ramesh, Journal of Energy Storage, 2021, 39, 102599.
- A. Halder, M. Aman, Lichchhavi, S. K. Jha, Journal of Electroanalytical Chemistry, 2024, 972, 118631.
- R. Balu, A. Dakshanamoorthy, Mater Sci: Mater Electron, 2022, 33,10057–10071
- M. Barazandeh, S. H. Kazem, Scientific Reports, 2022,12, 4628.
- H.Liu, R.Hu, J.Qi, Y.Sui, Y.He, Q.Meng, F.Wei, Y.Ren, Y.Zhao, W.Wei,Advanced Material Interface, 2020, 7, 1901659.
- S.J. Patil, J.H. Kim , D.W. Lee, Journal of Power Sources, 217, 342, 652-665.
- D.Shen,M.Y. Li,Y.Liu,X.Fu,H.Yu,W.Dong ,S.Yang, RSC Adv., 2023,13, 5557-5564.
- F. Zhao, W. Huang, D. Zhou, Journal of Alloys and Compounds, 2018, 755, 15-23.
- Z. Shi, X. Shen, Z. Zhang, X. Wang, N. Gao, Z. Xu, X. Chen, X. Liu,Journal of Colloid and Interface Science, 2021, 604, 292-300.
- J. Tang, W. Huang,X.Ly, Q.Shi, Nanotechnology, 2021, 32, 4817.
- N. V. Hoa, P. A. Dat, N. V. Chi, L. H. Quan, Journal of Science: Advanced Materials and Devices, 2021, 6, 569-577.
- S. Wang, P. Zhang, C. Liu, Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 616, 126334.
- Z. Kang, Y.Li, Y.Yu, Q. Liao, Z. Zhang, H. Guo, S. Zhang, J. Wu, H. Si, X. Zhang, Y. Zhang, Journal of Materials Science, 2018, 53, 10292-10301.
- Z. Ji, Z. Lin, J. Zhong, X. Gao, H. Li, G. Zhu, P. Song, X. Shen, Applied Surface Science, 2026, 718, 164902.
- X. Chen, M. Sun, F. Jaber, E. Z. Nezhad, K. S. Hui, Z. Li, S. Bae, M. Ding, Scientific Reports, 2023, 13, 15555.
- N. D. Hung, D. V. Cu, L.Q. Huy, L. T. T. Thuy, L. V. Khu, HNUE Journal of Science, 2025, 70, 88-100.
- H. Yu, D. Shen, R. Zhang, S. Zhao, Coatings, 2024, 14, 564.
- N. H. H. Yussuf, M. Z. Najihah, R. Hisam, H. J. Woo, M.F. Kasim, P. K. Singh, T. Winie, Research Square, 2025. doi.org/10.21203/rs.3.rs-7712061/v1.


You must be logged in to post a comment.