BY: VAIBHAVI MENON
“We are in this together and we will get through this together” is a quote we often hear in today’s time. The COVID 19 pandemic was definitely a situation that most of us didn’t expect and it resulted in a loss of thousands of lives and heart breaking situations. Coronavirus disease (COVID–19) is an infectious disease caused by a newly discovered coronavirus. The first COVID case was reported from Wuhan, China, on 31 December 2019. From there began the endless struggle to free the world of this hazardous disease and methods on how to do so are still being conducted as of today. The virus spread so quick that no one had time to prepare resulting in loss of economy and loved ones. The numbers kept increasing and it became hard to provide facilities for everyone in need of them.
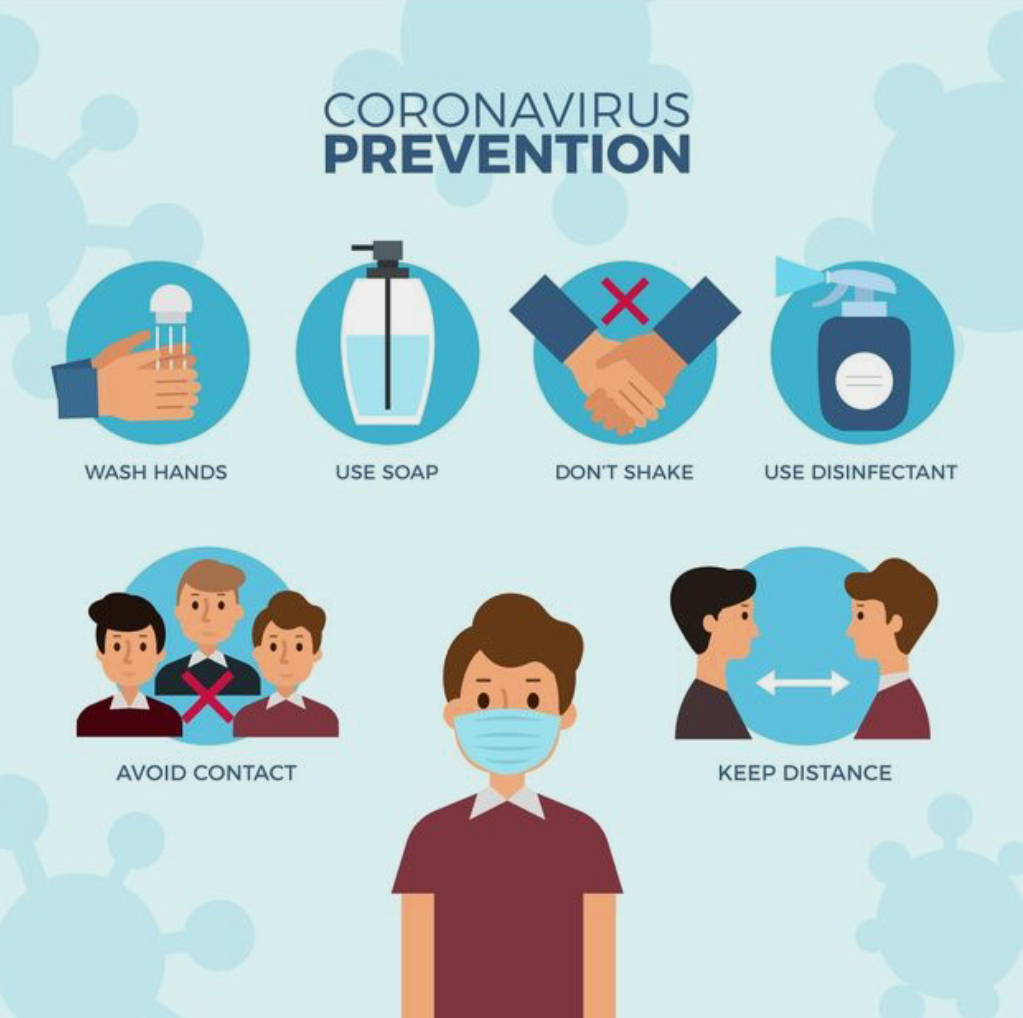
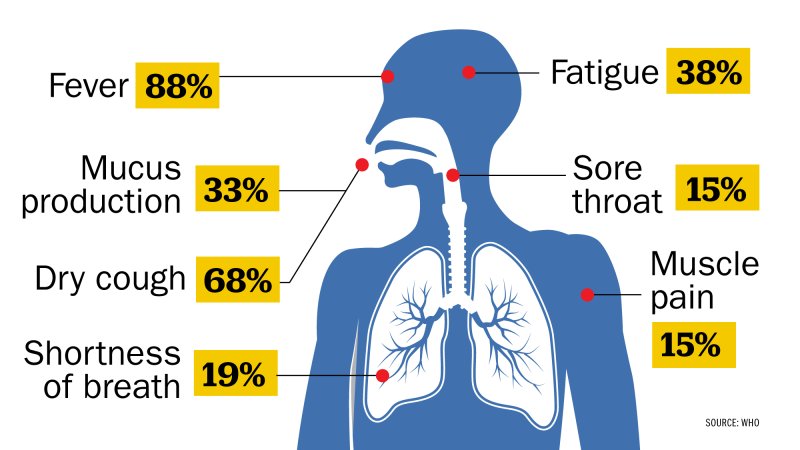
However ways on how to prevent the virus have definitely been helpful to a lot of us. One huge step taken was the world wide lockdown where people were asked to remain in quarantine and airplanes had shut down preventing travel of citizens to avoid spreading the virus. Prevention methods such as wearing of masks, maintaining social distance, avoiding social gatherings and even a nationwide lockdown were all put into place. Various testing methods had been developed to diagnose the disease. The standard diagnostic method is by detection of the virus’ nucleic acid by real-time reverse transcription polymerase chain reaction (rRT-PCR), transcription-mediated amplification (TMA), or by reverse transcription loop-mediated isothermal amplification (RT-LAMP) from a nasopharyngeal swab. Some common symptoms of the virus could be dry cough, fatigue or headaches while non common symptoms could be loss of taste and smell, breathing difficulties etc. Although the virus affected everyone in a negative manner, there were several companies who benefited from it and used it to their advantage. Companies such as Amazon, Zoom, Tesla, Apple had a rise in their economic prices. Vaccinations were soon introduced from different countries where some worked while some didnt and there was shortage in the number of vaccinations due to high demand for them. People also found new ways of communication through the technological platforms further helping in development of countries. Another positive aspect of this is that we as an individual have found time to spend with our family thus strengthening relationships and bonds with family. On the other hand communication has become more hander and children growing in this pandemic have tended to develop a introvert personality which could be a negative feature for them in the future.
In the present people have began to adapt to this form of lifestyle where technology plays a important role in survival and are slowly learning to be more comfortable with this method of communication. Inspite of that studies are still being made on how to put a stop to the virus or how to develop better methods of prevention which hopefully is something that’ll happen soon because We are in this together and we will get through this together.










You must be logged in to post a comment.