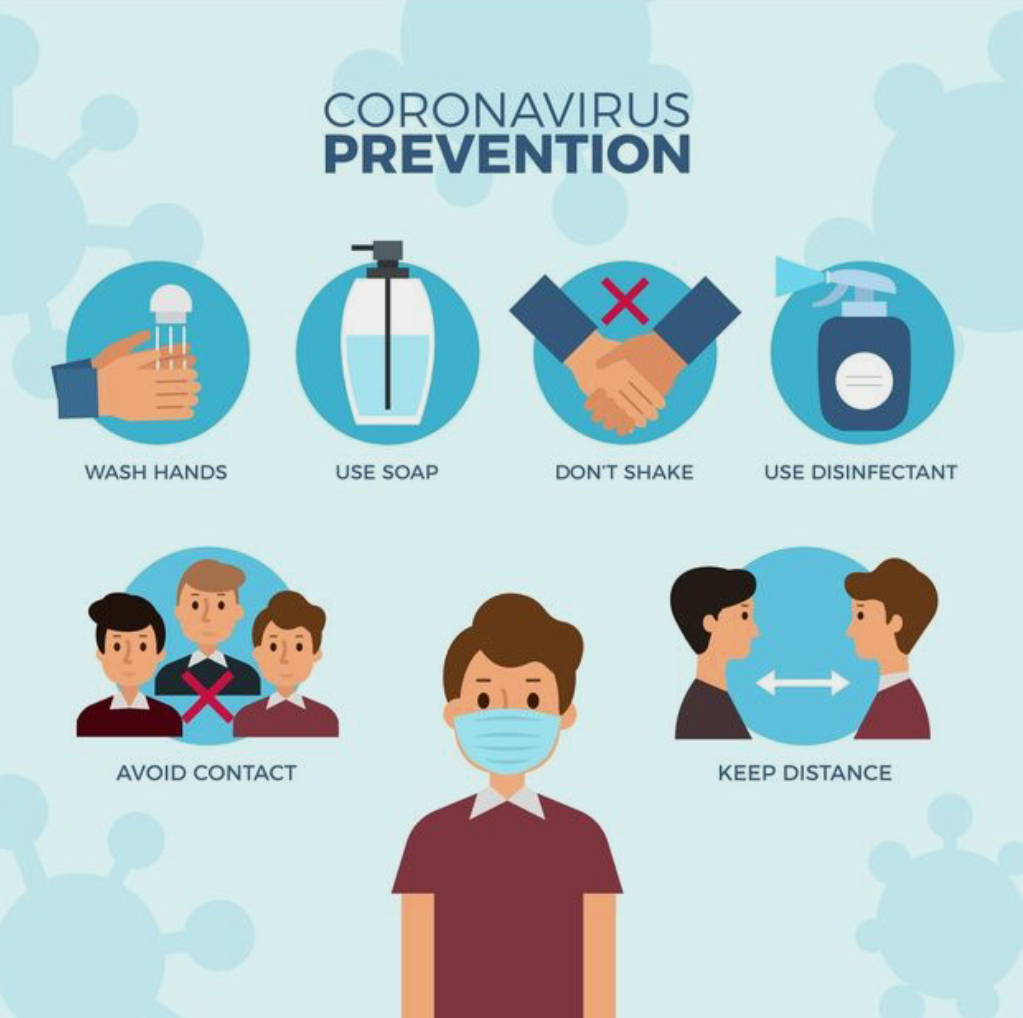
How long has it been since we went to school met our friends or had a family dinner at some restaurant? Since March 2020, we have all been advised to stay home and sanitise ourselves in order to stay safe. Sanitizers and masks have been added to our daily use products, all because of one tiny yet dangerous virus Covid-19!
The world is now waiting anxiously for a vaccine against this dreaded virus. Researchers around the world are working round the clock to develop vaccines to combat the pandemic. Currently, more than 165 vaccines against the corona virus are under process and 27 vaccines are undergoing human trials. Vaccines typically require years of research and testing before reaching the clinic. Today, scientists all over the world are racing to produce a safe and effective vaccine by next year.

• When did the efforts start
Efforts to make a successful Covid-19 vaccine began in January 2020 with the deciphering of the SARS-CoV -2 genome. The first vaccine safety trials in humans started in March but the road ahead remains uncertain. Some trials will fail and others may end without a clear result. But a few may succeed in stimulating the immune system to produce effective antibodies against the virus.
• India and vaccine against Covid-19
Like many other countries, India too is immersed in the efforts to develop successful vaccines to counter Covid-19. Thirty different Indian companies are trying to produce a vaccine to fight the infection. 7 out of these have received approval from the World Health Organization. These vaccines are in different stages of testing and clinical trials now.
COVAXIN
COVAXIN is developed by Hyderabad based Bharat Biotech International Limited in collaboration with ICMR and NIV, Pune. This is the first vaccine from India to get regulatory approval. The vaccine makes us an inactive version of a virus to spike up production of antibodies in the host body. It recently initiated Phase I and Phase II of clinical testing.
ZyCoV-D
ZyCoV-D is being developed by Zydus Cadila based in Ahmedabad. The Phase I trials of the vaccine have already begun. Extensive research was done regarding the same in collaboration with medical labs in Europe and US.

• Russia and vaccine against Covid-19
Russia is one of the countries worst affected by Covid-19. The country has been pushing extensively for a Covid-19 vaccine for quite some time now; Russia too is a part of the race to produce the world’s first Covid vaccine. It is possible that Russia would be ready with its first domestic corona virus vaccine soon. The clinical trials were conducted by the Gamaleya National Research centre of Epidemiology and microbiology. There seem to be no reports of side effects on the volunteers. All the participants showed immunity and the country is planning for the serial production of the vaccine by September. By the beginning of next year, Russia hopes to manufacture several million doses of corona virus vaccines per month.
• Oxford University’s efforts to develop a vaccine
The university of Oxford has partnered with AstraZeneca, a British-Swedish pharmaceutical company to develop an adenovirus vector vaccine to combat Covid-19. The vaccine prototype is currently in Phase III of testing. Trials of the vaccine developed by Oxford University show it can trigger an immune response. The vaccine which has so far been found to be safe and effective is expected to be made available for the masses by the end of 2020. This vaccine would also be the first such vaccine to have a large scale testing in India. The observatory data for this vaccine is expected to be available by November this year. The company has tied up with Pune based serum institute of India to mass produce the vaccine once the company gets required approvals and licensing from medical boards.

• Some methods used to make vaccines for Covid-19
Different scientists across the world try different techniques and formulas to develop vaccines. The Oxford researchers have put small sections of the corona virus genetic code into a harmless virus that infects chimpanzees. They appear to have developed a safe virus that looks enough like the corona virus to produce an immune system. Some other scientists have used pieces of raw genetic code, either DNA or RNA. When these are injected into the body it would start producing bits of viral proteins which the immune system can learn to fight. There is also work on corona virus vaccines called ‘plug and play’ vaccines. This method is new and less tested.
Thank you for reading. Have a nice day!🌼





You must be logged in to post a comment.