
“Strength does not come from physical capacity. It comes from an indomitable will.”
Mahatma Gandhi
Yes the will power should be strong and covid-19 checked it and many passed the test by accepting the lockdown situation during the crisis. The Covid-19 also known as Coronavirus is a group RNA virus which infects only mammals and birds. It causes respiratory related diseases which range from mild to lethal.This new group of viruses were named coronaviruses after their distinctive morphological appearance.Human coronavirus 229E and human coronavirus OC43 continued to be studied in subsequent decades. The coronavirus strain B814 was lost. It is not known which present human coronavirus it was.Other human coronaviruses have since been identified, including SARS-CoV in 2003, HCoV NL63 in 2003, HCoV HKU1 in 2004, MERS-CoV in 2013, and SARS-CoV-2 in 2019 which got spread worldwide, therefore named as Covid-19. This virus has made humans learn about humanity by helping others in pandemic situations. Many people lost their lives while fighting against the virus, due to lack of oxygen and treatment. The current 22nd June 2021 covid case study is total cases are 179,680,188 and deaths are 3,891,130 and the recovered ones are in no of 164,370,420. As the virus is mutating and evolving so vaccination is the key form to stop spread of this virus.
Vaccinate World
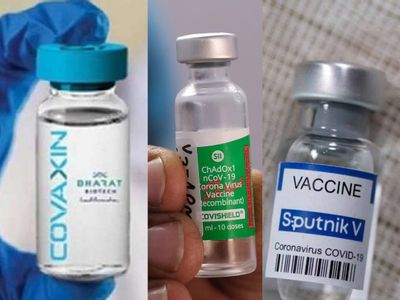
During Pandemic time it was the feeling that the whole world was a slave under coronavirus. But there were many frontline workers in the form of Freedom Fighters who fought for people and stood as a shield against deadly viruses . Many lost their lives but they fought till their last breath. Over 80 lakh frontline workers were lost in this fight. They are the doctors, nurses, the working and non-working staff of hospitals. They performed their duty without taking their own care. The only hope to stop covid-19 is Vaccine. Firstly, many researchers tested various mammals and studied its effects on them. Now the mission to Vaccinate world began. The vaccines were introduced around the world and each had a different time interval between doses. Here is the list of vaccines introduced in the world which gave hope to survive:-
1. Pfizer–BioNTech
2. Moderna
3. novirus vector vaccines
4. Oxford–AstraZeneca
5. Sputnik V
6. Johnson & Johnson
7. Convidecia
8. Sputnik Light
9. Sinopharm (BBIBP)
10. CoronaVac
11. Covaxin
12. Sinopharm (WIBP)
13. CoviVac
14. QazCovid-in
15. EpiVacCorona
16. ZF2001
Problems occurred and Faced them
We know that the world isn’t filled with only smart people, there are some people who are interested in troubling others. In this covid like situation some people became hurdle in path of the vaccinated world. Their bad deeds include selling oxygen cylinders in black, storing them in large numbers so as to sell at higher prices during shortages. They didn’t stop here, they even started a black market of vaccines. They know that poor ones are badly in need of vaccines so they take advantage of this situation and sell vaccines at 3 or 4 times higher price than the value set by the government and the poor ones have no option but to buy it. They are too helpless and no one comes to help them and they sell everything for a single dose of vaccine. But for bad people there are always punishers such as cops who made them come to the right path. The cops raided many places, seized vaccines and oxygen cylinders and arrested the gangs working behind these scams. All countries helped each other in one or other way and formed a special bond between them that is of humanity.
Spreading Awareness
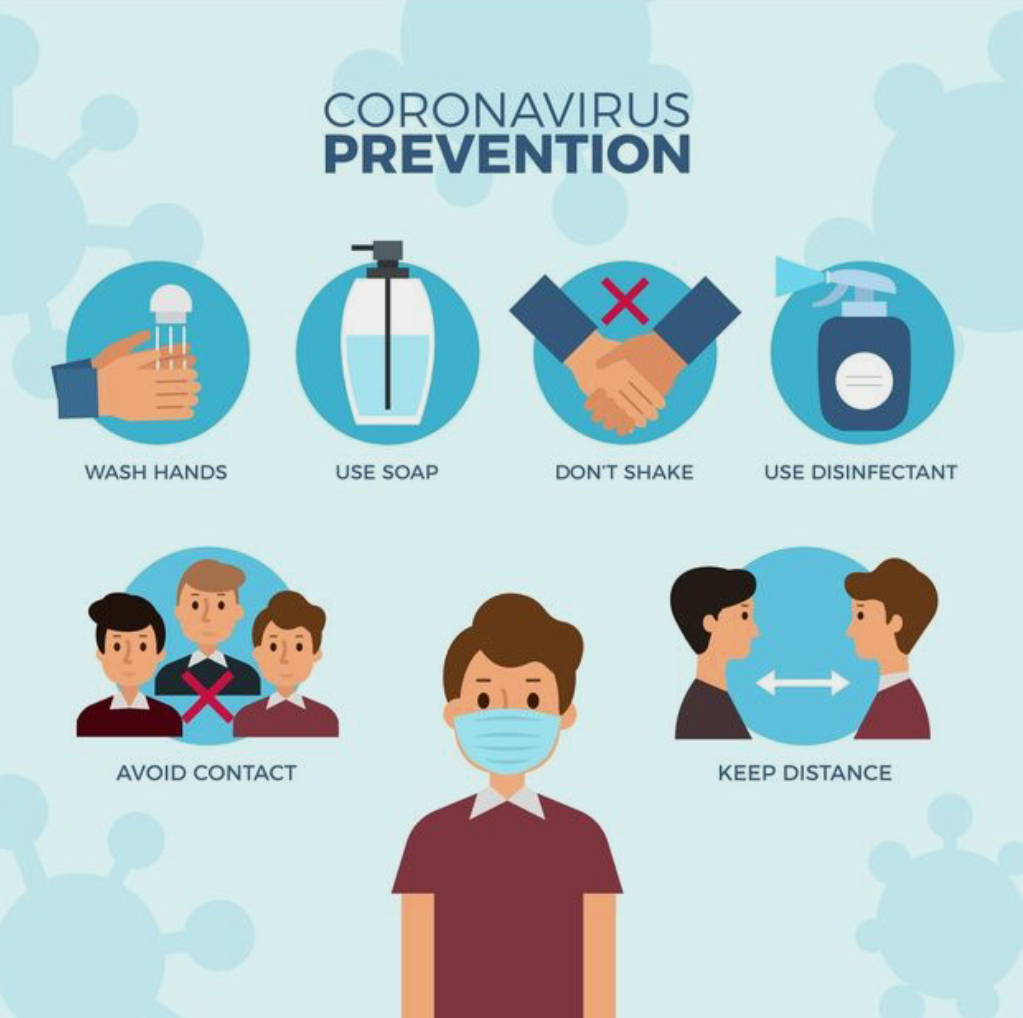
Social media, which is one the best inventions, can spread awareness among people regarding the use of vaccines. This awareness is much more important because many are unaware of the importance of vaccines. There are also some people who think doing vaccination is nothing, it is a waste of time. They also spread wrong information about vaccines. This information is spread within a minute in thousands through social media. This needs to be stopped. These bad influencers need to understand that vaccines are a type of advantage for people not a disadvantage. It is an essential key against covid virus. There are many good spreaders who understand and help people digitally through social media. I would specially refer to them as “digital frontliners”. So it is a humble request to spread awareness among people as much as you can.
At last I would like to say “Stay Strong Stay Safe”



















You must be logged in to post a comment.